Discovery & Development: Single-Stranded DNA
ssDNA: a novel approach to overcome efficacy and immunogenicity challenges in genetic medicines
What is single-stranded DNA and why is it a viable viral vector alternative?
Grant Boldt at CPTx
Recent advances in genetic medicine have made gene therapies and precise genome editing a clinical reality. However, persistent challenges in vector delivery, immune activation, safety and production scalability continue to limit broader impact. Conventional viral and double-stranded DNA (dsDNA) plasmid vectors often elicit undesirable immune responses, carry risks of insertional mutagenesis or face constraints on payload size. A compelling next generation alternative is single-stranded DNA (ssDNA), which is rapidly gaining recognition as a non-viral vector capable of delivering therapeutic genes and editing templates with enhanced safety, efficacy and manufacturability.
Challenges with conventional gene therapy vectors
Viral vectors – such as adeno-associated virus (AAV), lentivirus (LV) and retrovirus (RV) – are currently used in gene therapy but have limitations. AAV is limited by a small (~4.7 kb) packaging capacity, restricting the choice of therapeutic transgenes. Integrating vectors such as LV carries a risk of insertional mutagenesis, and clinical studies have demonstrated significant long-term safety considerations. Both viral and plasmid-based systems can trigger robust innate and adaptive immune responses, limiting repeat dosing and patient eligibility. Manufacturing these biologically based vectors involves complex cell culture systems, extensive purification and high production costs, impeding scalability.
Viral vectors also often require extensive engineering to reduce immunogenicity, which can compromise their transduction efficiency. Pre-existing immunity to common viral serotypes, such as AAV2, can significantly reduce therapeutic efficacy and exclude a large portion of the patient population. Furthermore, immune responses triggered by viral capsid proteins or vector DNA can lead to inflammation, cytotoxicity and clearance of transduced cells, especially in systemic delivery applications. These immune barriers complicate dosing strategies and often necessitate immunosuppressive regimens, which carry their own risks. Manufacturing viral vectors at clinical scale remains a major bottleneck. The production process involves transfection of producer cell lines, followed by multiple purification steps (such as ultracentrifugation or chromatography), each of which must be optimised for yield and purity. Batch-to-batch variability, contamination risks and the need for high-containment facilities further increase complexity and cost. These challenges collectively hinder the scalability and accessibility of viral vector-based therapies, particularly for large patient populations or repeated dosing regimens.1
ssDNA as a next generation vector
ssDNA, long DNA molecules composed of single nucleotide strands, is emerging as a powerful alternative. Naturally found in some viruses (eg, AAV, anellovirus), ssDNA in synthetic form offers flexibility in design, elimination of bacterial vector sequences and avoidance of dsDNA sensors. Long ssDNA constructs – scalable to 10-20kb – can encode therapeutic genes or editing templates without extraneous sequences, providing a clean and programmable genetic payload. Critical advantages arise from its structural simplicity; ssDNA is largely invisible to cytosolic DNA sensors such as the cGAS–STING pathway, reducing innate immune activation. Since ssDNA is non-integrating, it avoids the risk of insertional mutagenesis, and transient expression is possible where long-term gene expression is not needed. Designer sequence and modifications – such as removal of CpG motifs – can further reduce immunogenicity and improve in vivo stability.
Precision gene editing with ssDNA templates
ssDNA has proven to be a highly effective donor template for homology-directed repair (HDR) in CRISPR/Cas editing, as well as with other nucleases. Compared to dsDNA donors, ssDNA yields significantly higher HDR efficiencies and lower off-target integration in primary human cells. For example, it’s been demonstrated that T-cells edited with ssDNA templates achieved significantly improved viability and knock-in rates compared to dsDNA donors.2 More recent work reports HDR efficiencies exceeding 80-90% in primary cell types using optimised long ssDNA with Cas target regions engineered into the template.
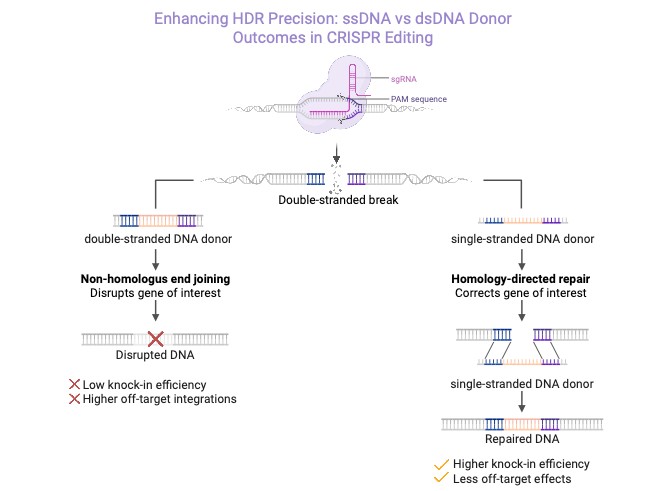
Figure 1: Enhanced CRISPR/HDR using ssDNA versus dsDNA donors
Mechanistically, ssDNA is more readily engaged by DNA repair machinery, avoiding triggering of DNA damage responses and reducing concatenation through non-homologous end joining (NHEJ). Modifications to the ends of strands further enhance HDR success. Circular ssDNA donors have also shown high efficiency in inserting large genetic cargos in immune cells, likely benefiting from enhanced stability and reduced exonuclease degradation.3,4 The dsDNA donor can provoke innate immune sensors and may integrate randomly, leading to lower on-target editing efficiency. In contrast, ssDNA donors pair seamlessly with the broken DNA ends, resulting in higher HDR efficiency, improved cell viability, and minimal off-target integrations (Figure 1).
Reduced immunogenicity and safety profile
The single-stranded structure of ssDNA allows it to evade cellular DNA-sensing pathways like cGAS-STING, which specifically recognises dsDNA, thereby avoiding inflammatory responses and toxicities in edited cells. Studies in haematopoietic stem cells and primary T-cells show lower cytokine induction and improved survival when ssDNA templates are used. ssDNA vectors lack viral capsids or protein components, eliminating pre-existing immunity issues and enabling potential for repeat dosing – a major limitation for viral vector therapies. Without random genomic integration, the risk of oncogene activation or tumour suppressor disruption is minimised. Furthermore, ssDNA designs can exclude immunostimulatory CpG to reduce TLR9-mediated immune recognition. These design features combine to create an immunologically ‘stealth’ vector, facilitating safer systemic or ex vivo administration.5
Scalable manufacturing and therapeutic feasibility
Manufacturing viral vectors remains expensive due to the reliance on cell cultures, complex purification and strict biosafety controls. While synthetic and enzymatic ssDNA production methods are advancing rapidly, an increasingly powerful and under-appreciated approach is microbial fermentation-based production of long ssDNA. This platform combines the biological scalability of microbial cultures with the precision of synthetic biology tools, offering several significant advantages for manufacturing genetic medicines (Figure 2). Fermentation systems – particularly those employing engineered E. coli strains – can be optimised to produce high yields of long ssDNA through phage-derived replication mechanisms or other recombinant processes. These microbial platforms harness the exponential biomass expansion capabilities of bacteria, enabling industrial-scale production at a fraction of the cost of enzymatic or chemical synthesis methods. Furthermore, ssDNA fermentation can be tailored to eliminate bacterial backbones and antibiotic resistance markers during purification, particularly important for in vivo therapeutic applications.6
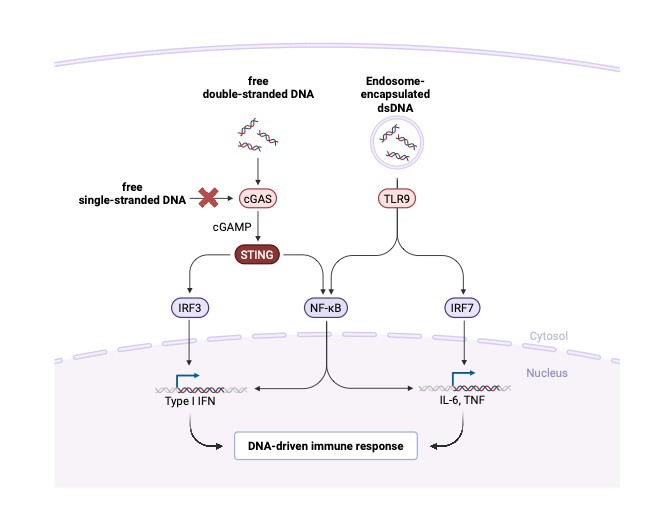
Figure 2: Immunogenicity comparison of gene therapy vectors
One key advantage of fermentation-based ssDNA manufacturing is high volumetric productivity, enabling multi-gram yields per batch. The systems are robust, reproducible and scalable using existing bioprocessing infrastructure, supporting rapid translation from lab-scale to good manufacturing practices-compliant manufacturing. Moreover, microbial ssDNA platforms can be programmed to produce customised sequences with integrated modifications (eg, defined ends, motifs for structural folding), expanding the diversity of therapeutic constructs that can be manufactured at scale.
As fermentation-based processes operate in living systems, they inherently allow for real-time quality control and metabolic optimisation during production. Integration of synthetic biology tools enables programmable control over ssDNA length, sequence composition and yield. These advantages significantly reduce the cost per base of ssDNA and facilitate broader access to this vector class for clinical and commercial use.7
To meet delivery requirements, long ssDNA products from microbial fermentation can be purified to high purity and formulated using lipid nanoparticles or alternative carriers. Microbial production can be adapted to generate circular ssDNA or hairpin-structured DNA for enhanced stability and expression duration. This flexible, scalable and cost-effective approach makes microbial fermentation an increasingly attractive manufacturing modality for ssDNA-based genetic medicines.
Emerging platforms and applications of ssDNA
Recent advances in synthetic biology and nucleic acid engineering have enabled novel applications of ssDNA beyond traditional gene editing. One promising area is episomal expression, where long ssDNA constructs remain extrachromosomal and drive transient gene expression without genomic integration. This approach is being explored for delivering therapeutic proteins and as a safer alternative to integrating vectors. In vaccine development, ssDNA is being investigated as a platform for encoding antigens and immunomodulatory elements. Its reduced immunogenicity makes it suitable for delivering nucleic acid vaccines with improved safety profiles. Studies have shown that ssDNA-based vaccines can elicit robust immune responses while minimising inflammatory side effects, particularly when formulated with lipid nanoparticles.
Another emerging application involves the use of ssDNA in conjunction with integrases – enzymes that mediate site-specific recombination. By designing ssDNA substrates compatible with integrase recognition sites, researchers aim to achieve targeted genomic integration with reduced off-target effects. This strategy holds promise for precise gene insertion in therapeutic contexts, such as engineering stem cells, and – given ssDNA’s immunological stealth – may address safety concerns for in vivo gene correction. Collectively, these applications highlight the versatility of ssDNA as a programmable and safe vector for diverse genetic medicine modalities.8,9
Outlook: towards safer, more effective genetic medicines
ssDNA is quickly becoming a versatile vector modality that addresses three major barriers in genetic medicine: efficacy; immunogenicity and manufacturability. As an HDR donor template, ssDNA enhances editing precision and cell viability. Its reduced interaction with immune sensors supports safer in vivo use and the potential for repeat dosing. Meanwhile, fermentation-based methods enable scalable, quality-controlled production of therapeutic-grade sequences.
It is anticipated that ssDNA based therapies – whether for genetic disease correction, in vivo gene editing or novel CAR T programming – will enter clinical trials in the near term. Each application may require tailored delivery methods or dosing strategies, but the foundational benefits of ssDNA remain consistent across modalities. In summary, ssDNA represents a promising vector platform uniquely suited to overcome long-standing limitations of traditional gene therapy vectors. Its combination of precision, reduced immunogenicity and scalable production positions ssDNA to play a pivotal role in realising the full therapeutic potential of genetic medicines.
References
- Mullard A (2021), ‘Gene therapy community grapples with toxicity issues, as pipeline matures’, Nature Reviews Drug Discovery, 20, 804-805
- Roth T et al (2018), ‘Reprogramming human T cell function and specificity with non-viral genome targeting’, Nature, 559(7714), 405-409
- Nitulescu A M et al (2025), ‘Single-stranded HDR templates with truncated Cas12a binding sequences improve knock-in efficiencies in primary human T cells’, Molecular Therapy: Nucleic Acids, 36(2), 102568
- Shy B R et al (2023), ‘High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails’, Nature Biotechnology, 41(4), 521-531
- Hopfner K P et al (2020), ‘Molecular mechanisms and cellular functions of cGAS–STING signalling’, Nature Reviews Molecular Cell Biology, 21(9), 501-521
- Kick B et al (2015), ‘Efficient Production of Single-Stranded Phage DNA as Scaffolds for DNA Origami’, Nano Letters, 15(7), 4672-4676
- Praetorius F et al (2017), ‘Biotechnological mass production of DNA origami’, Nature, 552(7683), 84-87
- Tang L et al (2023), ‘Circular single-stranded DNA as switchable vector for gene expression in mammalian cells’, Nature Communications, 14(1), 6665
- Tian Z et al (2024), ‘Circular single-stranded DNA as a programmable vector for gene regulation in cell-free protein expression systems’, Nature Communications, 15(1), 4635

As chief operating officer of CPTx, Dr Grant Boldt leads the company’s innovation-driven R&D in next-generation genetic medicines, guiding programs from discovery and design through delivery, manufacturing and preparation for first-in-human studies. His role integrates scientific vision with organisational leadership to advance pioneering approaches in genetic medicine. Dr Boldt joined CPTx in early 2024, bringing decades of experience at the intersection of biotechnology R&D and pharmaceutical manufacturing strategy. He previously served as vice president (VP) of Research Pharmaceuticals at Sigma-Aldrich, senior VP and business unit head of Biologics at Lonza and executive VP of Biologics at Avantor. Over the course of his career, he has authored numerous scientific publications and patents and has been a frequent speaker, with a consistent focus on translating innovative science into transformative therapeutic applications. Dr Boldt received his PhD in Chemical Biology from Scripps Research Institute, CA, US.