Manufacturing: Tabletting and Capsuling
Tablet density uniformity
Bill Supplee at Natoli Engineering discusses the best ways to ensure tablet density uniformity in pharma products
Many tabletting problems, such as poor friability and capping, are the result of density variation within tablets. Let’s look at some of the causal factors, how they affect tablet quality, and ways to optimise tablet and tool design to avoid this issue.
As compaction pressure is applied, a series of steps occur to form a tablet. First, particles will move, repositioning to take up less space. In this stage, most de-aeration occurs. At some point, particle rearrangement slows as no more efficient packing is possible and particles begin to deform at points of contact. As pressure increases, fragmentation and more deformation occur, both increasing the surface area of mating surfaces and bonding takes place. Deformation continues as a solid body until pressure is released, at which point decompression begins. While tablet compaction pressure is often treated as hydrostatic (such as for tip force calculations), powders are not fluids. A formulation’s particle size distribution can help or hinder the compaction process with larger particle sizes improving permeability. The formulation’s plasticity and ability to fragment affect bonding strength.
Tablet proportions play a key role. Aspirin was developed many years ago in a tablet that was too thin, which resulted in edge chipping issues and headaches for anyone who makes that product. A tablet that is too thin will tend to bond too quickly around the perimeter and restrict de-aeration. This then causes air to become trapped in the tablet and limits densification. As a result, the tablets will have a non-uniform density where the edges are very hard, but the center of the tablet will be soft. Hardness testing will show a low hardness necessitating higher compression force to achieve the tablet hardness required. These higher forces may sometimes exceed the punch tip force rating. Excessive forces can also lead to punch tip flaring and contacting the die walls. If this happens, it can seal off the de-aeration route, trapping more air in the tablet.

Tip flaring can crack or chip punch tips, and compensating for the reduced hardness by increasing the force can lead to over compressing the edges of the tablet, resulting in tablet lamination. Although the edges of the tablets are hard, the apexes of the cup formations and centre can remain soft, causing sticking problems and erosion.
Similarly, the cup profile is a critical aspect in tablet design related to tablet hardness uniformity. While deeper cup depths may be more aesthetically pleasing, easier to film coat and swallow with less edge chipping, they also reduce hardness uniformity and tip force rating. Cup profiles with radii that approach the land at steep angles pose additional challenges, as there is more particle movement during compaction along steep slopes.
Tablet size plays an important role in respect to air dissipation speed. Larger tablets have more air to be expelled with volume scaling cubically, while the overall size of the gap between punch tips and die bore does not. The average distance air must travel to escape also increases as tablet size increases. As bonding begins at the land area and moves up into the cup apex, the deeper the cup the longer this process will take. In many cases, tablet output is limited by the dwell time needed to compress the tablets or time needed to expel air. Tip clearances can be optimised as needed and die bores can be tapered to improve de-aeration. Pre-compression is often helpful in improving tablet density uniformity, as it is intended to de-aerate the powder bed.
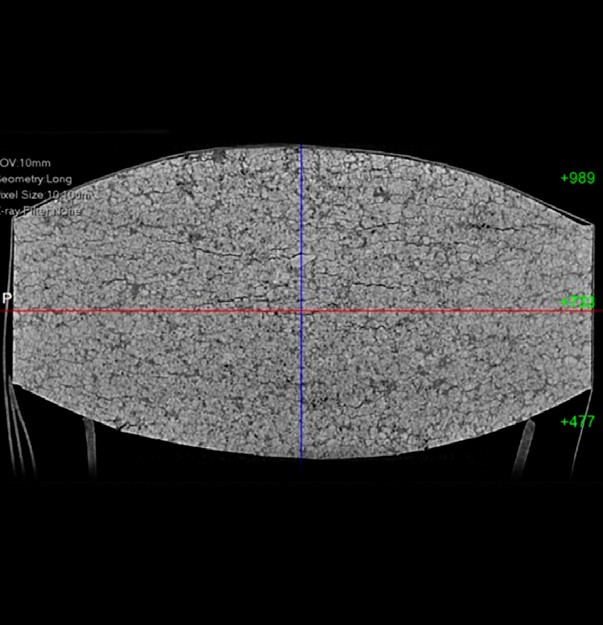
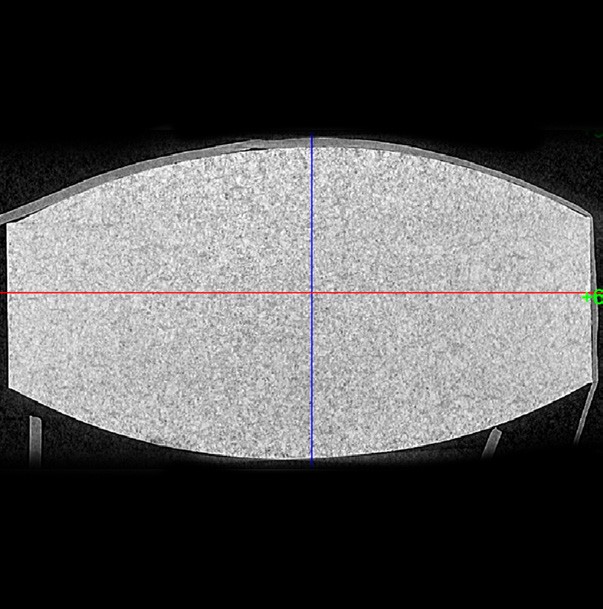
CT scans depicting less tablet uniformity (above) and more tablet uniformity (below)
Sizing your tablet correctly will become a little easier once the 8th edition of Tableting Specifications Manual becomes available. In it you will find a table showing the recommended tablet sizes for ranges of weight. While this is not going to be perfect, it will get you close. It’s best to work with your tooling vendor to develop the proper size tablet as they can model the expected tablet’s proportions.
A method for helping to determine the optimal tablet size would be to use tooling as close as you can get with tooling on-hand. Compress some tablets focusing on getting the desired hardness and thickness. The weight of the tablet is insignificant at this point in the process. The tablet thickness should be proportionally correct. You should find that adding or removing formulation, which will affect the band thickness, facilitates obtaining the desired hardness. Once these tablets are produced, weigh them and provide the thickness and weight data along with the tooling drawing you used to your tooling vendor. Let them know what your desired tablet weight should be and they will be able to calculate, based on the compressed volume, the best recommended tablet size for you.

If the tablets are already produced, a CT scan of them can show how uniform the tablet hardness is. This is a non-destructive method that develops cross-sectional images of the tablets internal structure. This is a new tool of the industry but very valuable in determining the cause of tabletting failures, as CT scans provide the ability to identify voids, cracks and delamination, as well as identify density inconsistencies.
Other benefits of CT scanning are to:
• Confirm uniform ingredient distribution in complex or fixed dose formulations
• Analyse content uniformity and detect high-density clusters or settling
• Preciselwy measure coating thickness and adherence
• Quantify uniformity of enteric coated tablets.
Whether you’re producing pharmaceuticals, nutraceuticals, confectionary or veterinary products, understanding your tablet’s mechanical properties allow for adjustment and optimisation. Tablet and tool designs can be customised to fit the needs of a product’s characteristics.


William “Bill” Supplee has over 50 years of experience in the pharmaceutical field working first with McNeil (J&J) for 32 years. He left that position to become a Manufacturer’s representative of pharmaceuti-cal tooling and processing equipment. After “semi-retirement” from sales, Bill took on his cur-rent role as Technical Sales Resource for Natoli Engineering.