Discovery & Development: Viral Clearance Studies
Viral clearance in advanced therapies
Should viral clearance studies be required for genetically engineered viral vectors and viral vector-derived products?
Tareq Jaber at Charles River Laboratories
Should the industry be running viral clearance studies for genetically engineered viral vectors and viral vector-derived products? The new version of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q5A stated that: ‘when appropriate, viral clearance (VC) studies should be performed to determine virus reduction factors for the relevant step(s) of the production process. The risk assessment for adventitious agents should be performed and evaluated on a case-by-case basis’. This statement indicates that, for late-phase studies, performing VC studies is not an option, however the requirements for each of these will vary based on the risk assessment of each product.
What does that mean and what should the industry do?
The guideline directs us to start with the general principles of viral contamination sources that we follow with other products, such as monoclonal antibodies (mAbs) and recombinant products. This includes:
Contaminations that could arise from a cell line
Usage of contaminated cells substrate and detection of latent/persistent viruses (such as herpesviruses) or endogenous contaminants (such as retroviruses). This point can be applied to gene therapy products using, for example, baby hamster kidney (BHK) cells (potential retroviruses contaminant), human embryonic kidney (HEK)293 cells (potential adenovirus contaminant) and SF9 cells (potential rhabdovirus contaminant).
Contamination that could arise from an adventitious viral contamination, either through:
• Contaminated raw materials/reagents, for example, cell culture (bovine viral diarrhoea virus/serum and mouse minute virus/medium), formulation and downstream purification
• Contamination caused by manufacturing operators or the environment (storage and handling). One of the major risks to assess is the production of gene therapy products at multi-product facilities and the risk of cross-contamination from other products that are produced at the same facility
• Contamination caused by using a virus or viral vector (including helper viruses used in their production).
What viruses to use?
One of the major targets that ICH Q5A (R2) highlighted for genetically engineered viral vectors and viral vector-derived products was to include a model virus’ representative adventitious, endogenous and, if possible, the relevant helper virus, in the VC study. In the rationale and action plan for the VC section, the new version of the guideline added a new ‘case’, Case F, to the other five existing cases. This case focused on helper virus, or viral vector for expression of recombinant protein, or virus-like particle (VLP), that is used in product manufacturing.
Several examples were cited in the document. First, the baculovirus/Sf9 system. Sf9 cell line is a Case C where the cells are known to contain a virus (rhabdovirus) for which there is no evidence of infectivity to humans. Virus removal and inactivation evaluation studies should use the identified virus. If it is not possible to use the identified virus, relevant or specific model viruses should be used to demonstrate acceptable clearance. In the case of Sf9, vesicular stomatitis virus is the common model virus that is used in VC studies. Baculovirus is mentioned in Case F as a source of contamination as a helper virus. Clearance of the virus should be demonstrated using the production virus or a specific model virus (eg, baculovirus). The second example is using mammalian cell lines such as HEK293 or BHK-21 with the presence of helper viruses such as adenovirus or herpesvirus (HSV). These cell lines were covered by Case B and D while adenovirus and HSV as helper viruses were covered by Case F.
What if it is a transient transfection process?
A risk assessment is still essential to justify and explain the low risk of potential viral contaminants for the transient transfection systems. This type of product is covered in Case A where no virus, no VLP, nor retrovirus-like particle other than the drug substance (eg, viral vector particles) has been demonstrated in the cells or the unprocessed bulk. Virus removal and inactivation studies should be performed with non-specific model viruses representing a range of different characteristics (enveloped/non-enveloped, RNA/DNA, high resistant/low resistant viruses).
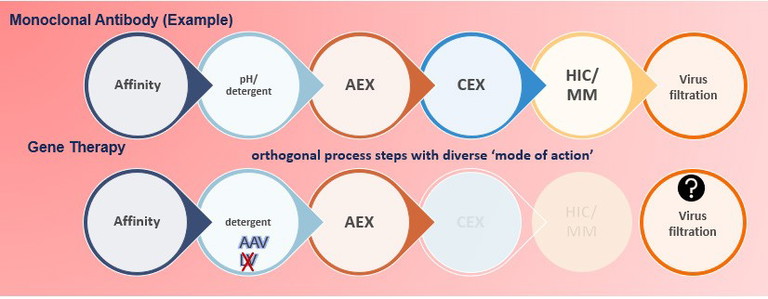
Figure 1: mAb versus gene therapy downstream steps
What is the expectation for a log reduction target?
Like all biologic products, acceptable log-reduction factors are based on risk assessment. According to the ICH Q5A (R2), whenever feasible, implementing two distinct VC steps – one of which should provide effective clearance of non-enveloped viruses – that complement each other in their mode of action is recommended. An effective VC step generally gives reproducible reduction of virus load in the order of, or more shown by, at least two independent studies. However, it is recognised that steps giving a reproducible reduction of one to three log10 contribute towards viral safety, and can be considered for assessment of overall virus reduction.
For viral vector products that are amenable to VC steps, the ICH Q5A (R2) does acknowledge that process steps tested in a VC study may not be as robust and efficient as recombinant proteins. In these cases, the viral safety of these products needs to be supported by a risk assessment, meaning that as such no four log10 target for at least one process step exists for viral vector products.
Downstream steps: which to test?
The typical mAb products have the ‘leverage’ to pick and choose from their downstream process steps to be tested in VC study. Gene therapy products don’t have this ‘leverage’ (Figure 1). There are several limitations that make the performance of VC study challenging and sometimes impossible.
Some of these limitations are:
• Downstream limited purification and polishing steps
• The product type, as since the product is a virus, some steps are not applicable for VC study. For example, while the traditional 20nm viral filtration step is considered to be the most robust step in monoclonal product studies, it cannot be used for gene therapy products where the product itself is a virus. Even though AAV systems can use larger nano-filters of about 35nm, these filters cannot retain viruses smaller than 30nm step, such as parvoviruses. Detergent treatment definitely cannot be used as a VC step for lentivirus nor herpesvirus systems since the viruses (the product) are sensitive to the detergent.
What are the common VC study test steps?
Affinity chromatography, anion exchange chromatography (AEX), viral filtration and detergent treatment are the most common steps used in VC.
“Virus safety should focus on testing and control of the raw materials and reagents entering the manufacturing process. The usage of well-characterised cell banks and virus seeds can reduce the risk of virus contamination”
Affinity chromatography and AEX have complementary roles in VC, with affinity chromatography being an effective purification step, rather than polishing. Conversely AEX is one of the most robust steps among all types of chromatography. For example, in Chinese hamster ovary-derived products’ VC studies, more than 65% of the runs for all four major viruses are above four log10 reduction.
Viral filtration is increasingly being recommended by regulatory agencies to increase the robustness of VC. Limiting for AAV, the nano-filter size that might be used in AAV production is not the traditional 20nm that is used for recombinant proteins, rather larger filter pore sizes (over 30nm) are being used during process development.
Already a very common step, detergent treatment is used to efficiently inactivate envelope viruses. Triton X, for a long time, was the main detergent to be used to lyse cells and consequently to inactivate any potential viral contaminant. However, in the past few years, due to environmental concerns, companies around the world have displayed considerable effort to find alternatives to Triton X. Recent testing has shown that several alternatives, such as Deviron, show promising results for inactivating enveloped viruses. However, not all products can accept or tolerate the same conditions, therefore it is important to test each product separately to ensure the best log reduction possible.
VC limitations: what are the alternatives?
Since the possibilities for applying VC steps during production are limited for many types of cell and gene therapy, the viral safety of these products should be ensured by applying a combination of measures.
For example:
• Virus safety should focus on testing and control of the raw materials and reagents entering the manufacturing process. The usage of well-characterised cell banks and virus seeds can reduce the risk of virus contamination
• Manufacturers should avoid using human-and animal-derived raw materials (eg, human serum, bovine serum, porcine trypsin) in their manufacturing processes
• Cell culture media or media supplement treatments such as gamma irradiation, virus filtration, high temperature short time processing or ultraviolet C irradiation can be used as additional virus risk mitigation measures
• Using closed processing, testing and other preventative controls.
As regulations continue to evolve, our VC study processes will have to adapt. Especially as new drug products are introduced, the industry will have to ensure that VC studies can appropriately identify virus reduction factors to ensure the safety of drug manufacturing and, ultimately, contribute to delivering safe, effective treatments to patients.

Dr Tareq Jaber is currently the associate director of the Process Evaluation group at Charles River Laboratories. He has been with Charles River since 2012, first working as a study director and then as a senior supervisor. He previously worked at the University of Pennsylvania School of Dental Medicine, Pennsylvania, US, in the Microbiology department as a postdoctoral fellow, and performed research on oncogenic herpesviruses. Dr Jaber received a PhD from the University of Nebraska-Lincoln, Nebraska, US, and his research focused on studying HIV pathogenesis and the discovery of novel proteins and small RNAs involved in herpesviruses latency stages.