Transforming R&D workflows with automated super-resolution microscopy
What is super-resolution microscopy and how is it accelerating progress across the imaging sector?
Anna Caballe at ACBio Marketing Consulting
Super-resolution microscopy has transformed life sciences research by providing nanometre-scale information on subcellular structures and uncovering crucial molecular mechanisms. New user-friendly platforms that integrate automated sample preparation, image acquisition and data analysis are making it a powerful and easy-to-implement tool. Super-resolution imaging is accelerating R&D, drug discovery and preclinical development, helping uncover drug-cell interactions, disease biomarkers and nanoparticle heterogeneity, with its use expanding to accelerate discovery pipelines and improve clinical outcomes.
How super-resolution microscopy works
Fluorescence microscopy allows scientists to selectively label and observe structures within cells to better understand physiopathological processes. In conventional light microscopy, structures below 200nm are ‘invisible’ due to the laws of diffraction. This limitation has been overcome over the last two decades through super-resolution imaging – various methods that act on fluorophore photoactivation or the excitation light to bypass the diffraction limit. Together with computational methods, super-resolution imaging results in images with nanometre-scale details and valuable outputs, including molecular morphology and spatial biomarker distribution.1,2 This groundbreaking technology is enabling scientists to study subcellular complexes with high sensitivity and nanometre precision, and understand single-molecule behaviour to unlock new discoveries in life sciences.
One of the pioneering super-resolution methods, single-molecule localisation microscopy (SMLM), can achieve sub-10nm resolutions by exploiting stochastic activation and deactivation of fluorophores. Transient ‘blinking’ events – where individual fluorophores emit light while neighbours remain dark – allow for single molecules to be precisely localised and subsequent data processing to yield molecular insights.3,4 SMLM techniques include: stochastic optical reconstruction microscopy (STORM), which achieves around 20nm resolution in fixed cells; point accumulation for imaging in nanoscale topology, which employs transient DNA-fluorophore binding for multiplexed imaging; and photoactivated localisation microscopy, which enables single-particle tracking in live cells through photoactivatable fluorophores.1-4
Electron microscopy, historically the gold-standard method for high-resolution subcellular visualisation, requires laborious sample preparation, where samples are studied after being removed from their native state. Super-resolution imaging allows for molecules to be studied in their native context, offering comparable nanoscale insights with improved molecular specificity and greater accessibility. Super-resolution is becoming a powerful alternative to elucidate molecular mechanisms inaccessible via conventional imaging, thanks to its capacity for quantitative outputs, which can lead to major breakthroughs in biomedical research.
Latest advancements in super-resolution microscopy
Super-resolution methods are accessible through commercial or open instruments and software. The high resolution and ease of implementation of new user-friendly platforms have boosted the adoption of SMLM by scientists of all skill levels. A crucial aspect of this progress is ensuring experimental data accurately reflects biology, free from system variations, particularly with biotechnology and pharmaceutical companies growing in users. The more robust systems integrate sample handling platforms to minimise user-led error, advanced imaging set-ups, and image processing and reporting to maximise resolution and precision when translating images into quantitative, actionable insights.
Early SMLM systems were only able to image a few cells at a time, resulting in low output numbers after long imaging sessions. The latest microscopes have expanded field-of-views, increasing imaging throughput to several cells or thousands of nanoparticles. Alongside tightly coordinated laser switching to enable rapid multichannel imaging, these systems allow for complex scientific inquiry by combining diffraction-limited imaging with super-resolution imaging.
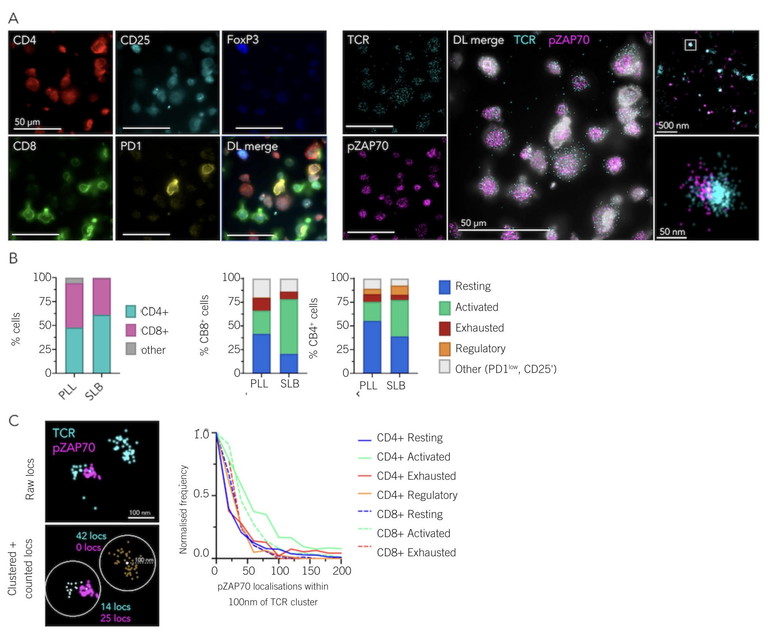
Figure 1: New super-resolution microscopes offer fast and precise multicolour multimode imaging that enables diffraction-limited imaging for cell classification and SMLM imaging for quantitative nanoscale information. A) Example field-of-view of five diffraction-limited (left) and 2 dSTORM (right) channels acquired. Zoomed regions correspond to boxes in larger images. B) Comparison of reported T-cell subpopulation frequencies on poly-L-lysine (PLL) and supported lipid bilayer (SLB) surfaces. PLL n = 326 cells, SLB n = 268 cells. C) Example of radial counting process used (left), and output histogram showing pZAP70 localisations per T-cell receptor cluster across different T-cell populations (right). Images were acquired using ONI’s Aplo Scope and analysed using ONI’s CODI software6
Multicolour multimodal imaging enables the detection and quantification of various markers, supporting colocalisation studies and the characterisation of heterogeneous populations with nanometre precision. This allows scientists to understand complex biological systems better, detect rare cell populations and analyse biomarker distribution (Figure 1). The ability to combine cell classification and super-resolution microscopy in a single assay unlocks the possibility to interrogate subtle nanoscale effects and spatial relationships within complex cellular contexts, which is fundamental to understanding diagnostic and therapeutic outcomes.5
The most disruptive innovation in the field has been the ability to package high power and precision in small yet stable instruments. Traditionally, super-resolution microscopes required dedicated dark rooms and vibration-free optical set-ups. New commercially available microscopes are uniquely engineered for stability and reliability, maximising reproducibility across experiments and users. With revolutionary designs, benchtop super-resolution microscopes have overcome the challenges of an ultra-compact design while providing precise temperature control, vibration-free integral isolation, sensitive next-generation cameras and safe laser enclosures, enabling versatile multimodal imaging workflows.
Quantitative super-resolution imaging to accelerate discoveries
Compared to conventional fluorescence microscopy, super-resolution imaging has become a highly accurate quantitative tool. The field has focused on developing single-molecule analysis algorithms that precisely capture the underlying biology, coupled with intuitive workflows for users to obtain reliable data with minimal training.7-10 The latest super-resolution platforms produce fast, reproducible and quantitative outputs for scientists to reveal how drugs interact with targets at the single-molecule level, identify prognostic disease biomarkers, uncover pathogenic mechanisms operating below the diffraction limit with nanoscale precision or characterise therapeutic nanoparticle formulations through individual particle measurements.
Super-resolution microscopy is now superior to traditional methods for obtaining multimetric molecular data. Instead of running separate experiments to correlate different metrics, it allows scientists to characterise multiple metrics in a single assay. This offers higher throughput and more comprehensive characterisation of samples. Super-resolution microscopy has positively impacted our understanding of biomarkers and pathogenic molecular mechanisms in complex diseases like cancer, neurodegeneration or infectious diseases. It has been used in different therapeutic areas, for instance, for guiding immunotherapy patient selection by exploiting ultra-low density antigens, understanding the mechanisms of thrombosis in cancer patients, detecting viral proteins in plasma, understanding aggregate biochemistry or the nanoscale reorganisation of neuronal receptors during pathogenesis, quantifying the distribution of key renal disease markers for a rapid and precise diagnosis, or assessing how biomaterials interact with cellular organelles ahead of clinical testing.11-15 Ongoing improvements in throughput and multiplexing – coupled with the integration of deep learning approaches and computational image analysis – are poised to further boost the use of super-resolution in drug discovery and development, clinical research, disease diagnostics and therapy optimisation.16,17
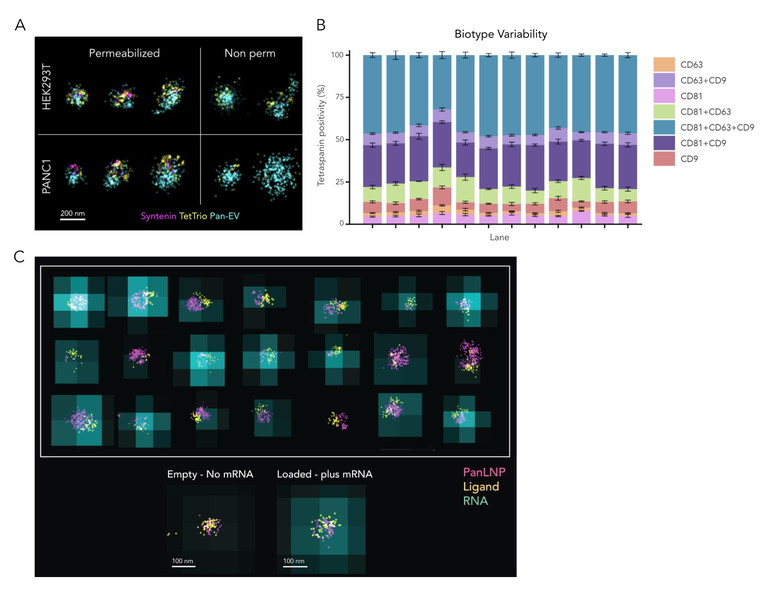
Figure 2: Automated end-to-end workflows allow scientists to accurately and reproducibly measure EV and LNP heterogeneity and function, ensuring reliable and quantitative insights into precious samples.7 A) Multicolour dSTORM combined with permeabilisation of EVs enables the detection of internal EV cargo (Syntenin). A membrane-based Pan-EV stain and CD81, CD63 and CD9 tetraspanin detection were used for EV identification and sizing. B) Automated sample preparation, image acquisition and analysis result in highly reproducible data. In this example, tetraspanin profiling with a st dev of less than 5% across three separate chips, chip lanes and field-of-views was obtained using ONI’s Aplo Flow, Nanoimager microscope and EV Profiler 2 Kit with AutoEV. C) LNP Profiling with SMLM and diffraction-limited imaging combined allows evaluation of LNP ligand engineering to accelerate LNP formulation optimisation and improve manufacturing output. Image taken using ONI’s LNP Profiler kit reagents, Aplo Scope microscope and AutoLNP software6
Drug discovery at the nanoscale
One promising application is the use of super-resolution microscopy for drug discovery. R&D labs are benefitting from precise and automated super-resolution imaging platforms to accelerate data-based decision-making throughout the drug discovery pipeline. With SMLM, molecular structures can be studied in detail to better understand drug-target interactions (both fixed and dynamic), as well as post-translational modificationdependent binding events or other physicochemical properties. Super-resolution imaging can provide unique insights into the mechanisms of action of complex medicines, result in higher sensitivity and specificity in clinical testing, and improve patient stratification for more personalised medicines.18
In recent years, super-resolution microscopy has proven its value in nanoparticle-mediated drug delivery. This class of complex medicine includes lipid nanoparticles (LNPs) and extracellular vesicles (EVs) able to protect and deliver therapeutic molecules to cellular targets of interest. Super-resolution microscopy allows for high-sensitivity visualisation and quantification of nanoparticles under physiological conditions, and enables researchers to precisely analyse crucial metrics like size, morphology and cargo loading at the single-particle level.19 Nanoparticle measurements at the molecular level with SMLM provide critical information, including biomarker distribution across subpopulations down to single particles (even those under 20nm in size), efficiency of cargo loading and ligand density (Figure 2). In addition, the compatibility of some super-resolution methods with live imaging can enable a better understanding of EV-mediated drug delivery and LNP uptake by recipient cells, allowing functional correlation studies.
Beyond high precision, super-resolution microscopy offers significantly improved throughput and efficiency. While gold-standard techniques like cryoEM may only analyse a few thousand particles per sample, SMLM processes over 100,000 particles, allowing for a comprehensive statistical characterisation of entire populations and allowing researchers to process a large number of samples in a single day with immediate results. The ability to quickly and accurately quantify metrics on a large scale is essential for evaluating the success of LNP formulations and intact purified EV preparations, which is critical to developing scalable and consistent manufacturing processes for therapies.
Conclusion
Single-molecule super-resolution microscopy is a highly sensitive and quantitative technology, with the ability to reveal subcellular mechanisms in live and fixed samples with nanoscale precision, detect pre-symptomatic disease biomarkers, characterise crucial drug-cell interactions and profile individual therapeutic nanoparticles. End-to-end automated solutions are now providing reliable and reproducible molecular insights across R&D workflows, helping to streamline the translation of bench discoveries into clinical pipelines.
References
- Kirti P et al (2022), ‘Super-resolution microscopy: a brief history and new avenues’, Phil Trans R Soc A, 380(2220), 20210110
- Jacquemet G et al (2020), ‘The cell biologist's guide to super-resolution microscopy’, J Cell Sci, 133(11), jcs240713
- Valli J et al (2021), ‘Seeing beyond the limit: A guide to choosing the right super-resolution microscopy technique‘, J Biol Chem, 297(1), 100791
- Lelek M et al (2021), ‘Single-molecule localization microscopy’, Nat Rev Methods Primers, 1(39)
- Lang F et al (2021) ‘Tackling Tumour Cell Heterogeneity at the Super-Resolution Level in Human Colorectal Cancer Tissue’, Cancers (Basel), 13(15), 3692
- Visit: oni.bio/aploscope
- Hugelier S et al (2023), ‘Quantitative Single-Molecule Localization Microscopy’, Annu Rev Biophys, 52, 139-160
- Wu Y L et al (2020), ‘Quantitative data analysis in Single-Molecule Localization Microscopy’, Trends Cell Biol, 30(11), 837-851
- Khater I M et al (2020), ‘A Review of Super-Resolution Single-Molecule Localization Microscopy Cluster Analysis and Quantification Methods’, Patterns, 1(3), 100038
- Dankovich T M et al (2021), ‘Challenges facing quantitative large-scale optical super-resolution, and some simple solutions’, iScience, 24(3), 102134
- Nerreter T et al (2019), ‘Super-resolution microscopy reveals ultra-low CD19 expression on myeloma cells that triggers elimination by CD19 CAR-T’, Nat Commun, 10(1), 3137
- Lucotti S et al (2025), ‘Extracellular vesicles from the lung pro-thrombotic niche drive cancer-associated thrombosis and metastasis via integrin beta 2’, Cell, 188(6), 1642-1661
- Singh R K et al (2023), ‘Detection by super-resolution microscopy of viral proteins inside bloodborne extracellular vesicles’, Extracell Vesicles Circ Nucleic Acids, 4, 557-67
- Brockmoeller S F et al (2025), ‘Single-molecule localisation microscopy (SMLM) is feasible in human and animal formalin fixed paraffin embedded (FFPE) tissues in medical renal disease’, J Clin Pathol, 78(5), 351-356
- Sun N et al (2023), ‘The power of super-resolution microscopy in modern biomedical science’, Advances in Colloid and Interface Science, 314, 102880
- Shi W et al (2023), ‘High-throughput single-molecule localization microscopy: Potential clinical applications‘, Clin Transl Med, 13(4), e1251
- Ma H et al (2020), ‘Super-resolution localization microscopy: Toward high throughput, high quality, and low cost’, APL Photonics, 5(6), 060902
- Toms L et al (2025), ‘Super-resolution microscopy as a drug discovery tool’, SLAS Discov, 31, 100209
- Alexandre L et al (2025), ‘Illuminating Extracellular Vesicles Biology with Super-Resolution Microscopy: Insights into Morphology and Composition’, ACS Nano, 15, 19(27), 24148-24173

Anna Caballe is the founder and CEO of ACBio Marketing Consulting, a Switzerland-based life sciences marketing and communications firm. Anna holds a PhD in Molecular and Cell Biology from King’s College London, UK. She specialised in super-resolution microscopy during her postdoctoral studies at the University of Oxford, UK, before joining Oxford Nanoimaging, where she developed her career in Product and Content Marketing Strategy, brand growth and technical writing.