Manufacturing: Advanced Contamination Detection
Why advanced contamination detection systems are essential for pharmaceutical manufacturing
What are eight compelling reasons why manufacturers should prioritise the use of advanced contamination detection systems in their operations?
Mike Pipe at Mettler-Toledo
Pharmaceutical production operates under some of the strictest quality expectations of any industry. Each stage, from formulation to packaging, demands absolute precision to protect patients and meet global compliance requirements.
Manufacturers face an increasingly complex set of challenges including cost pressures, shorter production cycles and growing regulatory scrutiny. Against this backdrop, physical contamination detection systems are no longer optional – they have become a critical component of an effective quality assurance programme.
Advanced physical contamination detection technologies such as metal detection and X-ray inspection deliver the confidence that pharmaceutical products are safe, compliant and market ready. These systems do far more than detect foreign body contaminants. They protect product integrity by performing completeness checks, such as confirming the correct number of tablets in blister packs or detecting product trapped in seals, while at the same time supporting the reduction of operational risks by identifying packaging defects early and improving efficiency through automated quality verification, minimising the need for manual inspection.
Below are eight reasons why manufacturers should utilise advanced contamination detection systems in their processes.
The detection of contaminants across multiple points
Contamination detection is fundamental to pharmaceutical quality control. Even trace amounts of foreign material can compromise patient safety and lead to significant financial and reputational consequences for the manufacturer. Metal detection technology provides highly sensitive inspection at critical control points in the process.
For example, a metal detection system installed before packaging can identify ferrous, non-ferrous and stainless steel contaminants in tablets and capsules early, preventing contamination from continuing down the production line. Metal detection can also take place even earlier in the process. Gravity fall systems, for instance, are used to inspect powders during active pharmaceutical ingredient production, reducing the risk of contamination before tabletting begins.
However, the challenge does not end there. Once products are packed in aluminium-based blister formats and sealed inside a final pack, X-ray technology can act as a second layer of protection. The technology identifies a wide range of contaminants including metal, glass, mineral stone and high-density plastics. X-ray systems operate by measuring variations in material density and can identify physical contamination regardless of shape, size or location within the product.
This dual inspection approach provides a strong foundation for physical contamination control throughout the manufacturing process. However, some manufacturers still have concerns about the use of X-ray technology. These misconceptions are just that. When X-rays are used, the radiation levels in pharmaceutical inspection are extremely low and applied for very short durations, meaning there is no impact on medication safety or efficacy. Extensive research confirms that X-ray inspection does not alter chemical composition or compromise the stability of pharmaceutical products. This makes it a safe and effective technology for achieving quality and compliance.
The protection of product and packaging integrity
Modern inspection systems extend beyond contaminant detection to verify that products and their packaging meet essential standards. X-ray technology can simultaneously perform multiple quality checks such as confirming the correct number of tablets or capsules in a blister pack, identifying damaged components, and ensuring that closures like screw caps are present and properly fitted.
Packaging integrity is also crucial. Although X-ray inspection cannot directly verify that seals are properly sealed, it can detect if product is trapped within the seal area, which may compromise the seal. Additionally, X-ray systems measure pack mass and confirm fill levels, helping to identify overfills or underfills that could affect dosage accuracy.
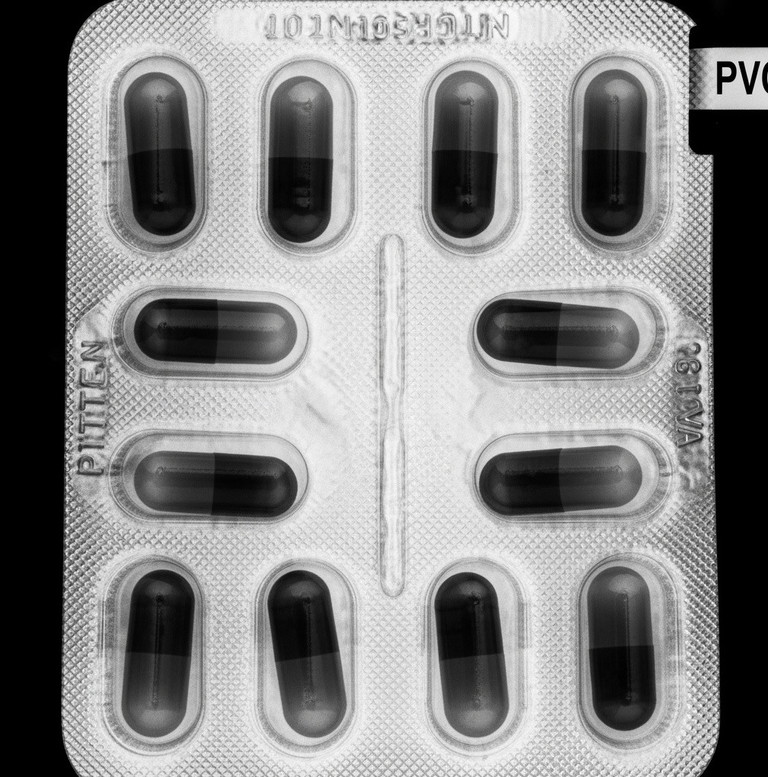
By integrating contamination detection with packaging integrity verification in a single system, manufacturers can ensure compliance while enhancing operational efficiency.
The avoidance of costly recalls
The cost of a product recall can be devastating. In addition to wasted materials and lost production time, there can be significant logistical costs and potential fines. However, the financial impact often pales in comparison to the damage caused to brand reputation.
By implementing a robust inspection programme that combines metal detection and X-ray inspection, manufacturers can significantly reduce the risk of defective or contaminated products reaching the market. Modern systems can assign run codes to each production batch, creating a complete digital record of inspection activity. In the rare event that a recall becomes necessary, this level of traceability allows manufacturers to isolate affected products quickly and recall only those batches rather than an entire production run.
The protection of your brand and reputation
Reputation is one of the most valuable assets a pharmaceutical company possesses. A single incident involving physical contamination can lead to public warnings, regulatory penalties and a loss of trust among healthcare professionals and patients. Regulatory bodies such as the US Food and Drug Administration (FDA) publish warning letters for non-compliance with standards such as FDA 21 CFR Part 210 (Current Good Manufacturing Practice in Manufacturing Processing, Packing, or Holding of Drugs) and 21 CFR Part 211 (Current Good Manufacturing Practice for Finished Pharmaceuticals), and these are publicly accessible. Such notices can damage industry standing and delay time-to-market for future products. Investing in reliable inspection technology demonstrates a commitment to quality and patient safety. Consistent product quality strengthens brand reputation and builds confidence across the supply chain. In short, contamination detection is not just about compliance, it is about protecting the future of the business.
The compliance with regulatory requirements
The pharmaceutical sector operates under some of the most stringent regulations in manufacturing. Compliance with good manufacturing practice and FDA requirements is essential. This includes measures to prevent physical contaminants from entering the product and to confirm that packaging remains intact throughout the supply chain.
Advanced contamination detection systems make this process easier. Features such as electronic signatures, operator login controls and time-stamped reports create a fully traceable record of each inspection. This digital audit trail helps manufacturers meet the requirements of CFR 21 Part 11 (Audit trails and digital signatures) and similar global standards, providing reassurance during audits and accelerating approval processes.
The combatting of rising costs
Cost pressures are a constant reality for pharmaceutical manufacturers. Rising raw material prices, higher energy costs and the need to reduce waste all contribute to the challenge. Effective inspection systems can help control these costs by minimising unnecessary rejects and preventing large-scale product loss. For example, a metal detection system with precise rejection capability removes only the contaminated product rather than entire groups of tablets, which significantly reduces waste. Similarly, X-ray systems identify packaging errors or underfilled products before they leave the factory, enabling the product to be reworked and improving overall yield. These efficiencies can deliver meaningful savings, particularly in high-volume environments.
The boost to productivity
Contamination detection technology is often viewed purely as a quality measure, but it also drives productivity. Systems that are simple to set up and easy to integrate into existing lines allow manufacturers to maintain high throughput without compromising quality. This is particularly valuable for contract manufacturers producing multiple products in short runs. The ability to configure contamination detection systems quickly for each new batch maximises valuable production time. Scheduled verification tests and condition monitoring functions further support efficiency by predicting maintenance needs and avoiding unplanned downtime.
The building of future-ready processes
The pharmaceutical sector is evolving rapidly. Trends such as personalised medicine, complex packaging formats and digital transformation demand greater agility and higher standards of accuracy. Advanced contamination detection systems position manufacturers to meet these challenges by integrating seamlessly with automated production environments and supporting real-time data analysis.
By investing in modern contamination detection technology today, manufacturers create a platform for continuous improvement. This not only protects patient safety and regulatory compliance but also provides the flexibility needed to remain competitive in an increasingly demanding market.
A combined approach for maximum protection
Metal detection and X-ray inspection should be viewed as complementary technologies rather than alternatives. Metal detection offers cost-effective sensitivity at early process stages, while X-ray inspection delivers versatility and additional quality checks at end-of-line. Together, they create a comprehensive inspection strategy that protects brands, improves efficiency and supports compliance.
Inspect. Protect. Comply.
Contamination detection technology is an investment in patient safety, regulatory compliance and operational excellence. Manufacturers who take a proactive approach to inspect their products can protect the future of their business by minimising risk, optimising productivity and complying with legislations.

Mike Pipe is head of Global Sales & Product Management at Mettler-Toledo. Over 15 years at Mettler-Toledo, Mike has worked in both commercial and technical roles helping food and pharma manufacturers and brand owners achieve compliance, maximise productivity and drive consumer safety. In this international strategic role, Mike works closely with local and global customers to understand their business challenges and applications in order to identify the correct x-ray technology solutions. His experience includes helping manufacturers to reduce the risk of product recalls, increase productivity and lower total cost of ownership.