Digital Health
Stepping Up the Pace in Vaccine Development and Production
Digitalising the entire vaccine development process can result in shorter timelines, allowing them to reach people faster, and with optimum quality
Andrew Whytock at Siemens AG

For the simulation process, the project partners had to decode the ‘black box’ of the formation of adjuvants’ particles. Image: Siemens
Vaccinations help protect against diseases and are one of the most important and effective preventive health measures available. The principle behind vaccination is to boost the individual’s own immune defences: vaccines expose the body to a minute quantity of pathogen-derived materials. The immune system responds by developing the appropriate immune cells and stores these protective cells as a kind of ‘immunological memory’. Not only does the vaccinated patient build up protection against the disease through vaccination, but in some cases, high vaccination rates among the general population make it harder for the individual pathogens to spread (called herd immunity), which may ultimately even result in their elimination.
The Long Journey of Vaccine Development
Developing vaccines is usually a costly and timeconsuming process. It always starts with biology – live samples or gene sequences of the pathogen. Researchers begin by analysing the components that are particularly important and, therefore, determining where an immune response could be effective. This can take up to several years, but is crucial to the vaccine development process and is followed by a series of stringent testing phases to confirm the safety and efficacy of the candidate vaccine. If the protection provided by the vaccine is proven and its safety profile is acceptable, the manufacturing company can then apply to register it. In parallel, the company develops the method to produce the vaccine in an efficient and robust way. It is a long phase that requires many experiments. It starts in the laboratory at a small scale: the process is designed, scientific knowledge is generated to understand the impact of all process parameters on product quality. Then the process is scaled up in a pilot plant to check its robustness, before transferring to the manufacturing site. The manufacturing plant prepares for mass production and sets up the appropriate production facilities.
Figure 1: The digital enterprise covers product design, in other words developing the vaccine (research and development), making the active ingredient (primary processing), and manufacturing the pharmaceutical itself (secondary processing). Image: Siemens
Digitalisation as an Acceleration Factor
Right now, vaccine development typically progresses in many small silos, each digitalised, to some extent, in its own environment, but with few connections between them. Often, one group depends on the completion of the work by another team to be able to access data. This is where there is potential for optimisation. Being able to consider the process as a whole, and digitalising the entire value chain, would represent a significant improvement – it would enable easier and earlier access to results, faster and more comprehensive feedback, better ability to predict and share outcomes, and provide better oversight of the entire process. Using a digital twin concept that combines the virtual and real worlds in a closed loop, it’s possible to test stages in the process and gain insights in a virtual environment right at the outset. Connecting the digital twin to a running process, it predicts the performance of the process, anticipates any deviation, and steers the control back to the optimal production. That allows vaccines to be developed faster and always be produced with the best information available. In addition, it helps ensure reliable supply. The data obtained from real runs is fed back with ML into the models, the ‘brain’ of the digital twin and, therefore, helps optimise both the digital twin and the products and processes from an early stage.
Case Study: Adjuvant Manufacture
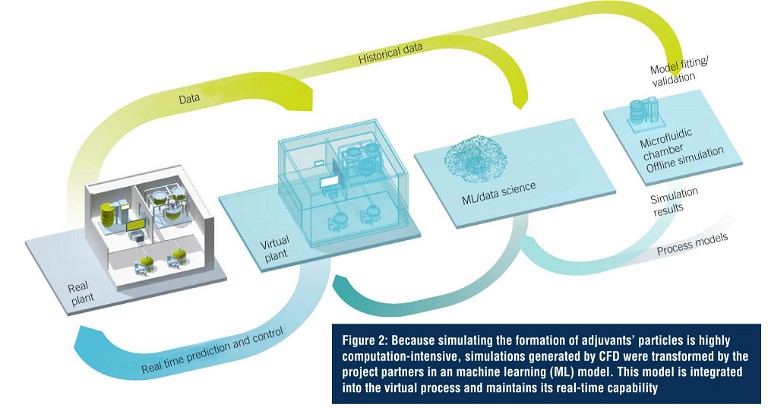
Adjuvants in the manufacture of its vaccines are additives that boost the immune response. This can play an essential role to help protect people with weaker immune systems (e.g., older adults or immunecompromised people) and can help reduce the volume of antigen required for each dose of vaccine, allowing the supply of more doses of the vaccine when demand is high. Testing the digital twin required a proof of concept, specifically for the development and manufacturing of adjuvant technologies. For the simulation, the ‘black box’ of adjuvants’ particles first needed to be decoded. Using mechanical models and artificial intelligence (AI), a hybrid model was developed to simulate and monitor the process. As such, the digital twin links the process parameters to the quality of the adjuvant. The sensors and PAT provide information that feed the twin to predict the quality of the product. Any deviation from the optimal quality is anticipated, and the twin acts on the process parameters to rectify and meet the target requirements. This digital twin is, therefore, based on simulation, AI, automation, and PAT. This makes use of various software solutions, and ensures unrestricted data transparency starting with product development and feeds the correlated data back into the process. An integrated platform that integrates hardware, software, and services facilitating complete access to the entire digitalised automation system and forming the basis for the engineering process used in implementation. Simulation software was used in process modelling and visualisation. The process is also supported by ML. The time factor, however, posed a particular challenge for adjuvant simulation.
Because the adjuvants’ particle simulation is highly computation-intensive, the computation process can take several hours. That’s a problem for real-time interaction between the digital twin and the real world. The project partners therefore extracted the process illustrated here and simulated it using computational flow dynamics (CFD). This enabled them to generate and save simulation files for all kinds of cases in advance. In combination with data from statistical trial planning and ML, this gives them the ability to predict the adjuvants’ particles that will be created with each change in critical parameters. As a result, the model is real-time capable. The digital twins for the next part processes will be developed as the project progresses.
Twins of the Overall Process
Digital twins of the product, production, and performance will be linked together, and offer particularly high added value in biological processes, or in cases where particular elements like physical models have to be better understood – as in the vaccines process. Quality management will be improved thanks to the use of soft sensors and process analytical technology (PAT).
With digital twins, it is now possible to collect data that help us understand exactly what is happening in real time during the vaccine production, enabling optimisation of operations. It allows not only monitoring of complex processes, but also predicts how changes would affect them. It allows researchers to run simulations in a matter of hours instead of building test plants. This new model – based on AI, ML techniques, and predictive and prescriptive models – provides new insights for development and full control over the vaccine production process. It enables a more robust process, delivering improved product quality – and ultimately helps deliver more vaccines, faster, to those who need them.

Andrew Whytock is Head of Digitalisation for the Pharmaceutical Vertical department in Siemens. He has global responsibility for digitalisation and innovation initiatives of Siemens in the pharma industry. His principal focus is to work with end users and partners to provide insight on Industry 4.0, Pharma 4.0, and digitalisation, facilitating their transition towards the digital pharma enterprise. One example is the recent collaboration with GSK and Atos in the optimisation of the vaccine development and production process. Andrew is an active member in both the ISPE Pharma 4.0 Special Interest Group and the Biophorum Technology & Digital Roadmap Steering Committees.