Imaging and Sensing: Mass Photometry
Analytics for a new therapeutic era: meeting the demands of modern biologics
As novel biologics reshape the therapeutic landscape, more advanced analytics are essential. Single-particle technologies can help fill this gap, delivering faster, clearer insights and supporting the next generation of advanced therapies
Salma Jalal and Jacintha Victoria at Refeyn
Biopharmaceutical pipelines are undergoing a fundamental shift, moving beyond monoclonal antibodies (mAbs) to include an expanding array of advanced therapeutic formats: viral vectors like adeno-associated viruses (AAVs) and lentiviral vectors (LVVs) are unlocking gene therapies for inherited and rare diseases; bispecific antibodies, antibody-drug conjugates (ADCs) and other engineered formats promise targeted therapies for cancer and autoimmune conditions; and lipid nanoparticle systems are broadening the toolkit for next-generation vaccines and therapeutics.
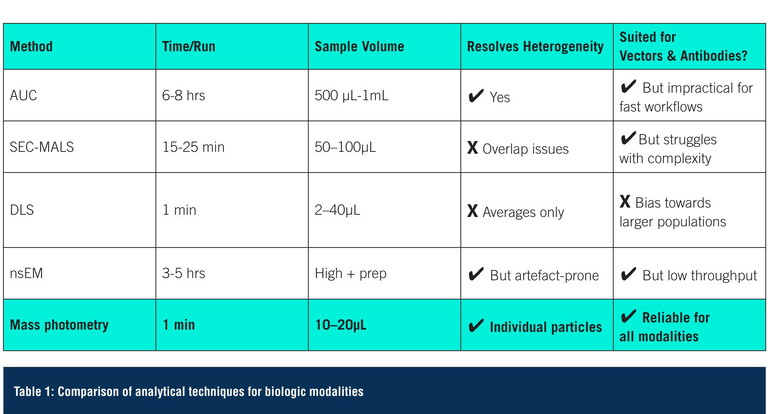
These novel modalities hold great potential, but bring a new tier of analytical complexity. Unlike traditional biologics, their inherent heterogeneity – differences in structure, payload content or assembly – can significantly affect therapeutic quality and patient outcomes. This complexity makes it difficult to generate meaningful data using traditional analytical methods. Established techniques like analytical ultracentrifugation (AUC), size-exclusion chromatography (SEC) and dynamic light scattering (DLS) struggle to deliver the speed, resolution and sample efficiency needed for detailed insights into composition, purity and structural integrity. As a result, there is growing interest in more agile, high-resolution, single-particle approaches like mass photometry and charge detection mass spectrometry (CD-MS) to meet the demands of this new era.
Analytical challenges for different modalities
As therapeutic modalities evolve, they each bring their own set of structural nuances and risks that require careful characterisation:
Viral vectors
Viral vectors underpin cell and gene therapies (CGTs), with AAV leading in vivo applications. Accurately distinguishing empty, partial and full capsids is critical, as the proportion of sequence-containing particles directly influences potency and regulatory approval.1 Degraded particles further complicate quality profiles. Analytical methods should deliver particle-level resolution under native-like conditions, along with straightforward workflows that return results quickly to guide process development and quality control. Similar demands extend to adenovirus (AdV) in vaccines and therapeutics, or LVV in engineered CGTs, where developers seek to characterise heterogeneous populations using minimal high-value material.
Antibody therapeutics
Bispecific antibodies and ADCs offer functional sophistication but also introduce distinct analytical challenges. Bispecifics form complexes with multiple binding stoichiometries, going beyond the 1:1 interactions seen with mAbs. For ADCs, variations in drug-to-antibody ratio and conjugation heterogeneity can significantly impact efficacy and toxicity. In addition to these modality-specific considerations, both formats must still meet standard quality criteria associated with mAbs, particularly around aggregation, which remains a critical attribute for ensuring safety and stability.
Complex biologics and delivery systems
Emerging platforms like lipid-based nanoparticles are advancing vaccines and nucleic acid therapeutics. However, they can be structurally fragile and inherently heterogeneous. Ensuring consistency in size distribution, payload, intactness and absence of impurities is critical. Across all modalities, developers face the same constraint: limited quantities of costly material. How can they achieve the necessary speed and resolution while conserving sample?
Current analytical landscape: what works, what doesn’t
Most analytical workflows still rely on a familiar set of legacy methods. These techniques can offer valuable insights, but can also have limitations for analysing today’s complex biologics.
AUC
A benchmark for assessing particle mass and composition, it is widely used for confirming AAV empty/full ratios. But it is inherently slow, often requiring 6-8 hours, large sample volumes and specialist expertise, making it better suited to orthogonal validation than agile process decisions.
SEC
Coupled to UV or multi-angle light scattering (MALS) detection, it is a mainstay for assessing size distribution and aggregation, but struggles to resolve co-eluting species, is sensitive to dilution effects, and can require significant method redevelopment (ie, column and buffer optimisation) for new samples and modalities. Mixed composition samples, like ADCs, can be challenging due to differences in UV absorbance between the protein antibody and non-protein payloads like small molecules and RNA.
DLS
This offers quick size assessments but averages the signal across populations, and so lacks the resolution to differentiate subtle species differences. Results can be biased by the presence of larger molecules or aggregates.
Negative stain electron microscopy (nsEM)
This enables qualitative assessment of particle morphology and is used to confirm capsid integrity in viral vector workflows. However, elaborate sample prep and non-native conditions mean it is impractical for high throughput or in-process use.
Surface plasmon resonance and biolayer interferometry
These help characterise antibody binding but can be hard to adapt for complex formats like bispecifics, where binding deviates from 1:1 stoichiometry. Surface immobilisation also limits physiological relevance, reducing suitability for detailed structural or compositional analysis.
Novel technologies bridging the gap: mass photometry
The limitations of traditional analytics have left space for new approaches designed to keep pace with complex molecular profiles. Across the industry, there is growing focus on methods that can deliver faster results, require smaller sample volumes, and resolve heterogeneous populations under native or near-native conditions.
This method detects individual molecules in solution based on the light they scatter as they interact with a glass surface. The interference between the scattered and reflected light corresponds to the molecule’s mass. This enables direct mass measurement at single-particle resolution without labels, columns or complex separations. The technique typically requires just 10-20µL of low-concentration sample and delivers results in one minute, providing rapid insight into molecular composition and heterogeneity under near-native conditions.
By resolving individual species, mass photometry can accurately distinguish intact proteins from aggregates, quantify the relative proportions of different oligomeric states and identify subtle shifts in sample quality that might be overlooked by bulk methods.2 This is especially valuable in complex antibody workflows, where aggregation, fragmentation and binding stoichiometry all impact product performance and safety.3
Mass photometry is also being increasingly adopted for analysis of AAV sample purity, particularly for quantifying empty, partially filled, full or overfull capsids with single-particle resolution. A closely related technique, macro mass photometry, extends these principles to larger viral particles. For each particle it measures both contrast (a proxy for mass) and size, enabling precise quantification of sample purity as well as empty and full populations of, for example, AdV samples.4 Together, these rapid, single-particle approaches offer a way to bridge the analytical gap for modern biologics, supporting faster, more informed decision-making across a broad range of modalities.
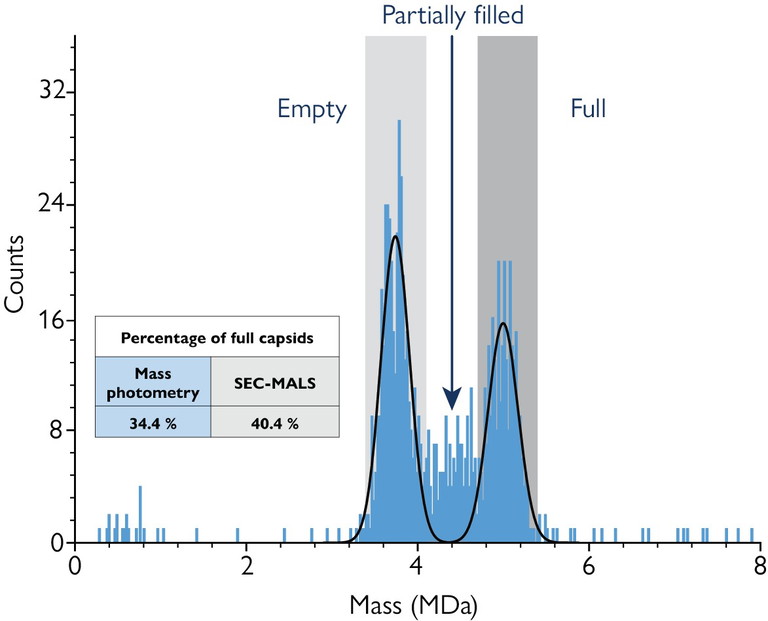
Figure 1: Mass photometry analysis of AAV capsids reveals distinct populations of empty, partially filled and full capsids based on individual particle mass. The measured proportion of full capsids (34.4%) closely aligns with values obtained by SEC-MALS (40.4%)
Performance and comparability to traditional methods
While established methods like AUC and SEC remain in use for analysing viral vectors and antibody products, they each have inherent limitations, whether in speed, sample demand or sensitivity to subtle heterogeneity (Table 1). Increasingly, mass photometry is being deployed alongside or in place of these approaches to fill critical gaps and provide complementary insight.
For AAV analysis, mass photometry aligns closely with established approaches like AUC and CD-MS in quantifying empty, partially filled and full capsid populations. Because mass photometry directly measures individual capsids, it resolves heterogeneous populations with high sensitivity.5
This single-particle resolution helps quickly detect quality issues – such as partially packaged or overfilled capsids (Figure 1) – that bulk measurements like SEC-MALS can miss. With a one-minute measurement time and sample requirements below 20µL, it makes process optimisation faster and more cost-effective.
In antibody analysis, mass photometry has demonstrated close alignment with SEC, widely used for monitoring aggregation. Side-by-side studies show that both methods yield closely aligned profiles for monomers and aggregates, confirming mass photometry as a valid orthogonal approach.6 Unlike SEC, it avoids dilution or shear-induced artefacts, and requires no re-optimisation when moving between antibody formats.
By filling analytical gaps left by conventional techniques, mass photometry brings speed, simplicity and sharper resolution of molecular heterogeneity, helping developers maintain quality standards while navigating tighter timelines.
Some application highlights:
• AAV gene therapies: mass photometry quantifies empty, partially filled, full and overfull capsids, supporting process optimisation and reducing variability ahead of regulatory filing (suitable for good manufacturing practice environments). It also enables upstream assessment of capsid purity, helping catch low-quality batches early
“By filling analytical gaps left by conventional techniques, mass photometry brings speed, simplicity and sharper resolution of molecular heterogeneity, helping developers maintain quality standards while navigating tighter timelines”
• Antibody therapeutics:mass photometry detects aggregation and can also resolve and quantify complex oligomeric forms under native conditions
• Larger vector platforms: macro mass photometry enables rapid checks on vector integrity and population distribution in vaccines and therapy workflows, enabling earlier process decisions and consuming minimal sample.
5. Gimpel A L et al (2021), ‘Analytical methods for process and product characterization of recombinant adenoassociated virus-based gene therapies’, Mol Ther Methods Clin Dev, 20, 740-754
6. den Boer M A et al (2022), ‘Comparative Analysis of Antibodies and Heavily Glycosylated Macromolecular Immune Complexes by Size-Exclusion Chromatography Multi-Angle Light Scattering, Native Charge Detection Mass Spectrometry, and Mass Photometry’, Anal Chem, 94, 892-900
Keeping pace with complexity
As biopharmaceutical pipelines evolve, analytics must keep pace. Single-particle technologies like mass photometry are helping developers meet the demands of increasingly complex biologics – delivering rapid, detailed insights from minimal sample under native conditions. By offering a clearer view of sample heterogeneity, purity and antibody binding earlier in development, these technologies support faster, more confident decision-making across everything from gene therapies to next-generation antibodies.
Ultimately, they help streamline processes and bring advanced therapies to patients sooner.
References
- McColl-Carboni A et al (2024), ‘Analytical characterization of full, intermediate, and empty AAV capsids’, Gene Ther, 31, 285
- Priest L et al (2021), ‘Scattering-based Light Microscopy: From Metal Nanoparticles to Single Proteins’, Chem Rev, 121, 11937-11970
- Wu D et al (2020), ‘Measuring the affinity of protein-protein interactions on a single-molecule level by mass photometry’, Anal Biochem, 592, 113575
- Young G et al (2018), ‘Quantitative mass imaging of single biological macromolecules’, Science, (1979), 360, 423-427

Salma Jalal is a market development manager at Refeyn, supporting biopharma customers in advancing analytical workflows through mass photometry. She holds a PhD in Mechanobiology from the National University of Singapore, Singapore, and has over a decade of experience applying advanced microscopy to biological systems.

Jacintha Victoria, a market development manager at Refeyn, has more than ten years’ experience in biotechnology and gene therapy. She holds an MSc in Cellular and Molecular Biology from the University of New Haven, CT, US, and is certified as a technologist in Molecular Biology by the American Society of Clinical Pathology.