Imaging & Sensing: LVEM
LVEM: a new tool for AAV gene therapy and pharmaceutical development
How is low voltage electron microscopy revolutionising the electron microscopy landscape – making the practice more accessible in pharma labs?
Daniela Vieira and Jared Lapkovsky at Delong Instruments
The adeno-associated virus (AAV) is now an ‘industry-standard’ gene therapy vector.1 AAV particles (around 25nm diameter) must be tightly controlled in manufacturing due to their key critical quality attributes, including identity, potency, purity and, particularly, the ratio of genome-containing (‘full’) to empty capsids. A low fraction of full capsids forces higher dosing and increases immune risk.1-3Consequently, robust analytics are required throughout development of AVV particles.
The traditional cryo-transmission electron microscopy (cryo-EM) technique is a gold standard for measuring full/empty populations, but this is costly and slow to access. In practice, the high expense and specialist expertise needed for cryo-EM, typically operated at energies over 100kV, make routine quality control (QC) difficult.3 Recent work shows that low voltage electron microscopy (LVEM), which is operated at energies under 25kV, and is typically available in benchtop and compact form factors, can achieve 1.0nm resolution with sufficient image contrast to recognise and characterise virus and virus-like particles (VLPs).3,4 These developments open a new avenue to inhouse and compact transmission electron microscopy (TEM) for viral vector QC.
LVEM: principles and advantages
LVEM employs accelerating voltages between 5-25kV (versus 100-300kV in standard TEM). These reduced energies allow for the construction of benchtop and compact microscopes. Low voltage electron microscopes operated at 25kV have been shown to provide sufficient resolution to resolve 20-30nm virus and nanoparticle drug carriers. The slower electrons have stronger interaction with the sample, giving superior image contrast to their higher energy counterparts – a boon when imaging unstained biological particles – making viral capsid interiors (empty versus full) distinct.
Importantly, LVEM systems are far cheaper, smaller and easier to operate than conventional TEM systems.5 Benchtop and compact instruments require only a normal lab outlet and no dedicated infrastructure, such as cooling water and vibration isolation. In short, LVEM democratises EM for more labs.
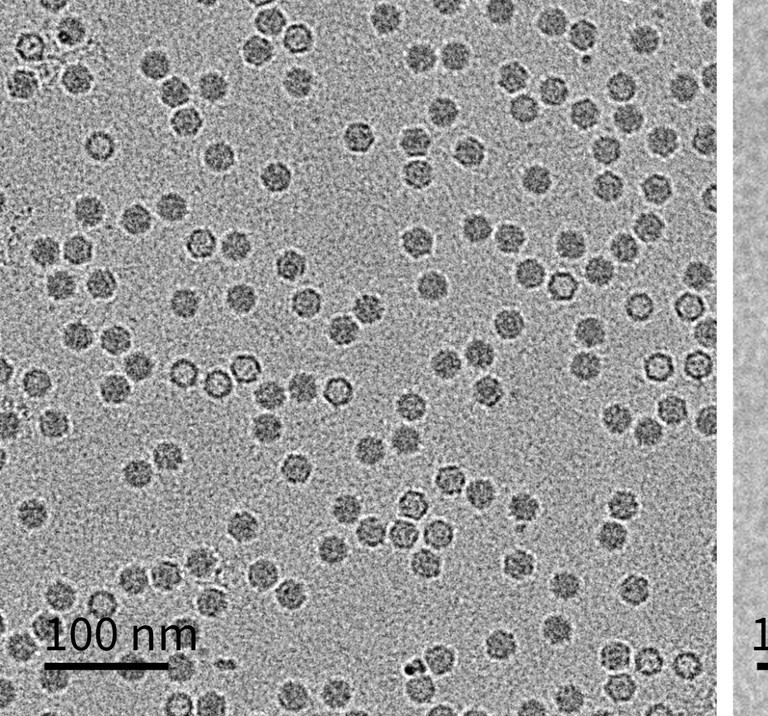
LVEM in AAV Characterisation
The practical utility of LVEM for characterising AAV particles was recently demonstrated in a study where unstained TEM grids of AAV were imaged in an LVEM and analysed with neural-network software.3 LVEM clearly distinguished empty capsids (dark centre) from full capsids (light interior) due to Fresnel fringes under defocus. By counting thousands of particles, the LVEM-derived empty/partial/full (EC/PC/FC) populations agreed closely with orthogonal methods (sedimentation velocity analytical ultracentrifugation and charge-detection MS). In practice, the entire LVEM workflow (staining, imaging, artificial intelligence-based classification) took only a few hours per sample, much faster than sending the samples out to a third-party analysis lab for cryo-EM service. The LVEM results from an unstained sample protocol were found to be more conclusive than using standard staining protocols, as stained empty AAV particles would occasionally erroneously show as full when in fact they were suffering from stain-impregnation (Figure 1). Using LVEM also provided unique structural insights; using negative staining, incomplete or ‘broken’ capsids became visible. The study observed that such fragments often appear as regular dimers or trimers, as if partial capsids cluster to ‘passivate’ exposed edges.3 These clusters – along with larger aggregated species – were readily identified in LVEM images. In other words, LVEM enabled visualisation of high-molecular-weight impurities and morphological defects that are critical quality attributes (CQAs) for AAV preparations.
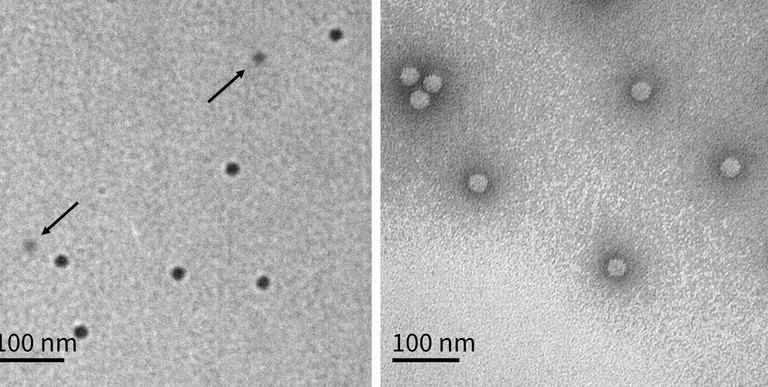
Figure 1: TEM Method Comparison. (a) Typical cryo-TEM image. Full capsids have dark centres, while empty capsids have light centres. (b) Typical unstained LVEM image. Full capsids are dark circles, while empty capsids are lighter circles indicated with arrows. (c) Typical stained LVEM image. Images are of the same model system B sample. All scale bars are 100nm. Reproduced from Ausman et al (2025)3
In summary, LVEM has proven capable of measuring the most important AAV CQAs in one place: particle count; full/empty ratio; and presence of aberrant particles. By bringing these analyses in-house, companies can accelerate development and improve batch-to-batch control.
Broader pharmaceutical and nanomedicine applications
LVEM’s utility extends well beyond the characterisation of AAV particles. TEM is already recognised as a gold standard for characterising nanoparticles and VLPs in vaccines and biologics.6 However, conventional TEM has been impractical for many manufacturers (especially SMEs and labs in low-resource settings) due to cost and complexity. Low-voltage systems aim to change that.5
LVEM also complements other QC tools. For instance, where dynamic light scattering (DLS) or high-performance liquid chromatography give bulk measures of size, TEM provides direct images. LVEM can clearly differentiate between two distinct particle size populations and/or agglomerated particles, while DLS would not be able to.
In practical terms, the LVEM can be applied wherever nanoparticle size and morphology are critical. This includes lipid nanoparticles for RNA vaccines, polymer or inorganic drug carriers, exosomes and VLP-based biologics. As one literature example, a LVEM was used in scanning electron microscope (SEM) mode at 5kV to image silver nanoparticles embedded in a polymer hydrogel for antibacterial gels.7
Similarly, academic and industry groups have begun using low-voltage EM to detect adventitious viral contaminants or protein aggregates in biologic drug formulations, tasks once only possible with large TEMs. In short, the LVEM offers a practical bridge: bringing high-resolution imaging in-house for researchers to use as they need. By combining ease-of-use with modern image analysis, LVEM can help biopharmaceutical developers incorporate EM-based assays as routine QC, not just one-off research.
“LVEM systems make gold standard EM accessible for nanoparticle drug delivery, biologics development and vaccine production”
Conclusion
LVEM is emerging as a critical tool for pharmaceutical innovation. For AAV gene therapies, LVEM enables in-house measurement of capsid integrity and payload content, at a fraction of the cost and complexity of cryo-TEM. More broadly, LVEM systems make gold standard EM accessible for nanoparticle drug delivery, biologics development and vaccine production. As the gene therapy field grows, analysts will need faster, more flexible assays for emerging modalities. By combining compact hardware with high-contrast imaging, LVEM is doing just that. In our view, these tools are poised to democratise EM in the pharma lab – accelerating development and ensuring the quality of advanced therapies.
References
- Naso M F et al (2017), 'Adeno-Associated Virus (AAV) as a Vector for Gene Therapy', Biodrugs, 31(4), 317-334
- Wörner T P et al (2021), 'Adeno-Associated Virus Capsid Assembly Is Divergent and Stochastic', Nat Commun, 12(1), 1642
- Ausman K D et al (2025), 'Low Voltage Electron Microscopy: An Emerging Tool for AAV Characterization', J Pharm Sci, 114(3), 1554-1562
- Möller L (2020), 'Diagnostic Electron Microscopy of Viruses With Low-Voltage Electron Microscopes', J Histochem Cytochem, 68(6), 389-402
- Vieira D et al (2025), 'Low-Voltage Electron Microscopy (LVEM): Part I — Benchtop and Compact TEMs with Heightened Contrast for Soft Materials', Microsc Today, 33(4), 17-20
- González-Domínguez I et al (2020), 'Quality Assessment of Virus-Like Particles at Single Particle Level: A Comparative Study', Viruses, 12(2), 223
- Rodríguez Nuñez Y A et al (2019), 'Preparation of Hydrogel/Silver Nanohybrids Mediated by Tunable-Size Silver Nanoparticles for Potential Antibacterial Applications', Polymers, 11(4), 716

Daniela Vieira is an applications engineer at Delong Instruments (Delong America). With over 15 years of experience in materials research and nanotechnology, Dr Vieira specialises in the characterisation of nanomaterials and biological samples using advanced analytical techniques (TEM, SEM, scanning transmission electron microscopy, energy dispersive X-ray spectroscopy, electrochemical tools, X-ray diffraction, Fourier-transform infrared spectroscopy, etc). She holds a Bachelor’s degree in Materials Engineering, a Master’s in Bioengineering, and a PhD focused on biomarker detection using nanotechnology and electrochemical methods. Her expertise includes the development of novel nanomaterial applications, the enhancement of imaging techniques for biological specimens and effective collaboration within multidisciplinary teams to address complex scientific challenges. In addition to her research background, Dr Vieira has experience providing training and technical support to new users of advanced microscopy systems.

Jared Lapkovsky is international sales manager at Delong Instruments (Delong America). In his role, Jared demonstrates a profound expertise in the applications of LVEM, leveraging his 15 years of experience. With a deep understanding of the technology’s capabilities, Jared adeptly communicates the benefits of LVEM to clients across diverse industries, including materials and life sciences, as well as clinical applications. His strategic guidance and customer-centric approach enable organisations to harness the power of LVEM for advanced imaging and analysis, revolutionising their research and development processes.