Discovery and Development: GalNAc
GalNAc: game-changers in developing liver-targeted conjugate therapies
How have N-acetylgalactosamine conjugates changed the way liver-related diseases are treated?
Hansjoerg Streicher and Adrian Hery Barranco at Biosynth
Targeted drug delivery to the liver is essential for treating a range of liver-related diseases, including viral hepatitis, liver cancer and metabolic disorders. However, conventional therapies often suffer from poor specificity, leading to off-target effects and suboptimal therapeutic outcomes. GalNAc (N-acetylgalactosamine) conjugates offer a promising solution as they provide a selective, efficient method of reaching hepatocytes, making them a valuable tool in modern therapeutic development. This approach enables precise hepatic targeting with fewer off-target effects, and could reshape the development of liver-specific therapies.
The growth of GalNAc clusters
The use of triantennary N-acetylgalactosamine (triantennary GalNAc or triGalNAc) conjugates for targeted drug delivery is driving significant growth in liver-targeted therapeutics. Projections for the whole liver cancer therapy market indicate around a 19% compound annual growth rate (CAGR) through 2035, according to industry analyses.1 Recently valued at $3.1bn in 2023, the market is expected to expand significantly as more therapies advance through clinical pipelines.1 This growing interest is not only driven by clinical demand for effective liver-targeted therapies, but also by a deepening understanding of the molecular interactions that make GalNAc conjugates so effective. At the heart of their success lies the biology of carbohydrate–receptor interactions, particularly with the asialoglycoprotein receptor (ASGPR), a C-type lectin primarily expressed on the surface of hepatocytes that governs targeted uptake in liver cells.2
Carbohydrate-receptor interactions
Carbohydrate-receptor interactions play a key role in how cells recognise each other and their environment, which has been vital to the evolution of complex life, especially in mammals. Dysregulation of these interactions can contribute to a range of chronic liver-related diseases, including non-alcoholic steatohepatitis (NASH), liver fibrosis, cirrhosis and hepatocellular carcinoma, as well as genetic disorders that can be addressed using RNA-based therapies like small interfering RNA (siRNA). These interactions are central to glycoprotein clearance, immune signalling and cellular communication. A common feature of these interactions is multivalency, where multiple sugars (oligosaccharides) on a cell’s surface (the glycocalyx) bind to several carbohydrate-recognition sites on one or more proteins. Similarly, cells can display many copies of a carbohydrate receptor on their surface to selectively recognise soluble sugars or the glycocalyx of another cell, such as the ASGPR.3
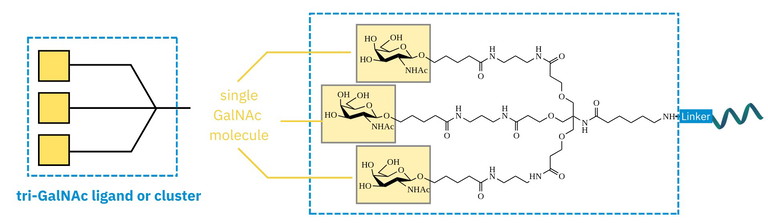
Figure 1: Representation of a GalNAc cluster ligand (left) and one example (tri-b-GalNAc-6-aminohexanoate, right)
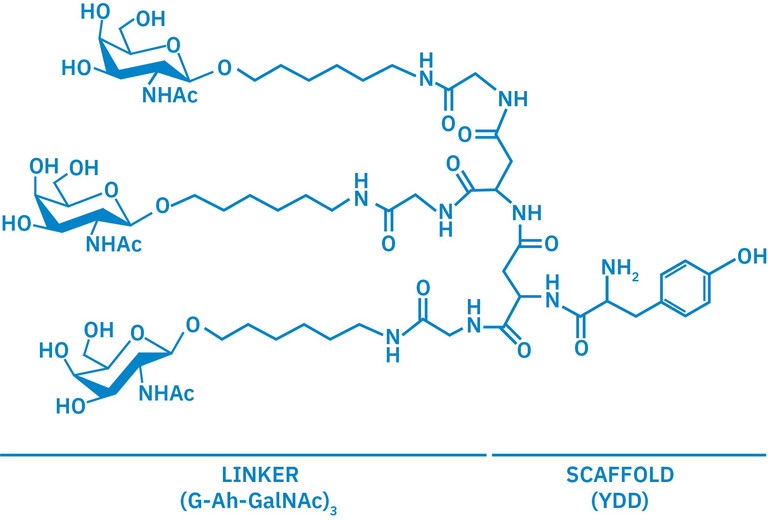
Figure 2: Structure for YDD(G-ah-GalNAc), a peptide-based scaffold used to present a trivalent GalNAc cluster in a specific spatial arrangement optimised for binding to the ASGPR. YDD is a tripeptide composed of tyrosine (Y) and two aspartic acid (D) residues that serves as the central core or backbone of the scaffold; (G-ah-GalNAc)₃ are the three flexible branches, each made of glycine (G), aminohexanoic acid (Ah) and terminal GalNAc unit
Early studies showed that individual carbohydrate-protein interactions tended to be highly specific but weak in strength. However, a critical breakthrough came in the 1970s and 1980s when researchers discovered that clustering multiple copies of a carbohydrate could significantly enhance overall binding strength through a phenomenon known as the avidity effect.4 To leverage this, researchers explored presenting several copies of the same sugar on synthetic scaffolds. These sugar clusters (if arranged correctly) could bind simultaneously to multiple receptor sites to significantly boost overall binding strength. Based on this phenomenon, clusters of GalNAc have become powerful tools in drug development, evolving over the past few decades into key components of promising liver-targeted therapies.5-7
A GalNAc cluster refers to a small group of GalNAc residues arranged in a trivalent configuration (Figure 1) that together promote high-avidity binding to ASGPR. These trivalent clusters have become the gold standard for achieving efficient hepatocyte targeting.
The ASGPR, also known as mammalian hepatic lectin (HL), is a type II transmembrane glycoprotein consisting of a cytosolic domain, a transmembrane domain, a stalk region and a C-terminal C-type lectin domain. This C-type lectin domain is a 135-150mer polypeptide and binds its sugar ligand, which is galactose or N-acetylgalactosamine (Gal/GalNAc) in a calcium-dependent manner. Following Gal/GalNAc binding, the ligand is internalised and separated in the endosome of the liver cell.
Designing and optimising GalNAc clusters for precision liver targeting
The success of GalNAc-conjugates in liver-targeted therapy stems not only from their selective interaction with the ASGPR, but also from the careful design of the carbohydrate clusters themselves.
Mammalian HLs have two highly homologous subunits – HL-1 and HL-2 – that exist in close proximity to each other, both being required for high sugar affinity and effective internalisation. The rigid organisation of HL-1 and HL-2 to form a trivalent binding site results in an affinity enhancement 103-105 compared to monovalent Gal/GalNAc-binding (the Kd of which is in the millimolar range), when a ligand offering three Gal/GalNAc residues, such as a desialylated glycoprotein, is bound. However, this ‘glycoside cluster effect’ can only occur (or reach a maximum) when the spacing between the sugar residue is optimal.5
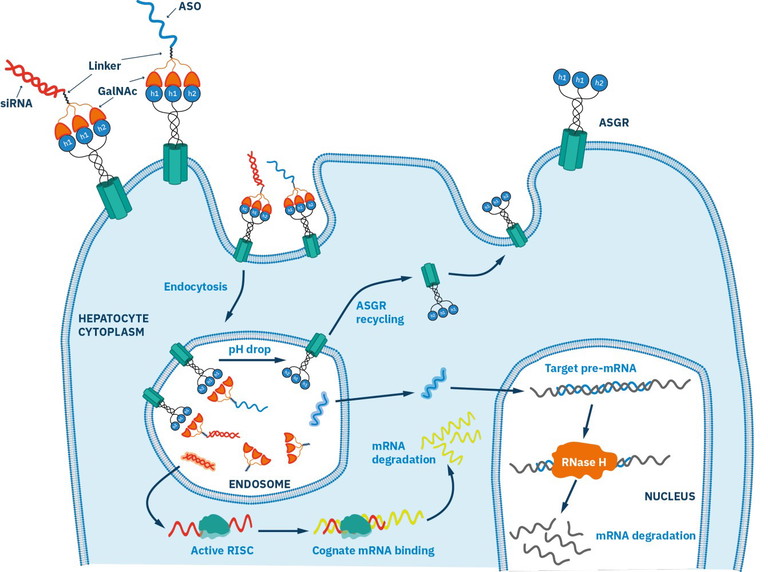
Figure 3: Schematic diagram of GalNAc-mediated siRNA and ASO delivery to liver hepatocytes. The trivalent binding site of ASGR formed from the HL-1 and HL-2 subunits are fully occupied by the trivalent targeting clusters
A key element is valency, which refers to the number and spatial arrangement of GalNAc units on a given scaffold. Studies have shown that trivalent clusters (typically bearing three GalNAc residues) offer an optimal balance of binding strength, specificity and synthetic accessibility, making them the go-to design for clinical applications.5-9
A wide range of scaffolds have been developed to present GalNAc units, including triGalNAc, in a biologically favourable orientation. Early designs include peptide-based cores such as YDD(G-ah-GalNAc)₃ (Figure 2), which remain a reference in cluster architecture.5 Alternatives like tris(hydroxymethyl) aminomethane, or dendritic frameworks based on branched amino carboxylic acids, allow researchers to fine-tune the presentation of GalNAc clusters. These AB₃-type scaffolds enable high-affinity binding to ASGPR, and provide flexible options for attaching therapeutic payloads or additional targeting elements.9
One major application has been in antisense oligonucleotides (ASOs). These short, chemically modified strands of DNA bind to RNA and regulate gene expression. Conjugation of ASOs to a trivalent GalNAc cluster has been shown to significantly improve potency and duration of effect by enhancing uptake through ASGPR-mediated endocytosis.10 The resulting GalNAc–ASO conjugates act as prodrugs, selectively releasing the active ASO inside liver cells after internalisation.
Mipomersen (Kynamro), an early ASO treatment for homozygous familial hypercholesterolaemia, was one of the first to utilise this targeted delivery approach.11 Although it was withdrawn from the US market in 2019 due to concerns over hepatotoxicity and cardiovascular events, it was one of the first successful examples of GalNAc-based targeting, demonstrating its potential to improve drug delivery and therapeutic outcomes for liver-related diseases.12
Direct therapeutic ‘warhead’ delivery
This molecular engineering has enabled the delivery of various therapeutic ‘warheads’ directly to hepatocytes. siRNAs have also been successfully delivered using the same strategy. These molecules work by harnessing the natural RNA interference (RNAi) pathway to silence specific genes (Figure 3). A hallmark of this therapeutic class is givosiran (Givlaari), a GalNAc-siRNA conjugate used to treat acute hepatic porphyria approved in 2019.13 It targets ALAS1, a key enzyme in heme biosynthesis, and prevents the build-up of toxic precursors in liver cells.
Through ASGPR-mediated uptake, givosiran delivers its siRNA payload directly to hepatocytes, enabling targeted gene silencing with high efficacy and precision. Prior to its approval, treatment options for acute hepatic porphyria were limited to intravenous haemin therapy and symptomatic management, both of which had significant limitations and often failed to prevent recurrent attacks.14-16
Liver-targeted drug delivery redefined
The combination of well-engineered GalNAc clusters and efficient receptor-mediated uptake has created new possibilities in hepatic drug delivery. These innovations have transformed previously challenging therapeutic approaches – such as gene silencing and RNA-based therapies – into viable clinical strategies, establishing GalNAc conjugates as true game changers in liver-targeted drug development. The field of liver-targeted drug delivery has seen remarkable progress in a short span of time, much of it driven by the thoughtful design of GalNAc clusters and their strategic pairing with RNA-based therapeutics. What began as an insight into sugar-protein interactions has grown into a powerful toolkit for tackling some of the most challenging liver diseases. GalNAc-ASO and GalNAc-siRNA conjugates have already redefined what is possible in liver-targeted drug delivery, proving that with the right molecular design, even the most challenging therapeutic goals can be met.
References
- Visit: snsinsider.com/reports/liver-cancer-therapeuticsmarket-3215
- Crocker P R et al (2007), ’Siglecs and their roles in the immune system’, Nature Reviews Immunology, 7(4), 255-266
- Morell A G et al (1968), ’Physical and chemical studies on ceruloplasmin: V. Metabolic studies on sialic acid-free ceruloplasmin in vivo’, Journal of Biological Chemistry, 243(1), 155-159
- Cummings R D et al (2022), ’Chapter 25 C-type lectins’, Essentials of Glycobiology, 4
- Lee Y C et al (2000), ‘Molecular Characteristics of Hepatic Lectins in Carbohydrates in Chemistry and Biology’, Wiley-VCH, 4, 549
- Sharon N et al (2004), ’History of lectins: from hemagglutinins to biological recognition molecules’, Glycobiology, 14(11), 53R-62R
- Martínez-Bailén M et al (2023), ’Multivalent glycosystems for human lectins’, Chemical Society Reviews, 52, 536-572
- Debacker Alexandre J et al (2020), ’Delivery of oligonucleotides to the liver with GalNAc: from research to registered therapeutic drug’, Molecular Therapy, 28(8), 1759-1771
- Müller C et al (2016), ’Organizing multivalency in carbohydrate recognition’, Chemical Society Reviews, 45(11), 3275-3302
- Prakash T P et al (2014), ’Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice’, Nucleic acids research, 42(13), 8796-8807
- Bennett CF et al (2010), ’RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform’, Annual Review of Pharmacology and Toxicology, 50, 259-293
- Visit: pmc.ncbi.nlm.nih.gov/articles/PMC11022857/
- Visit: fda.gov/drugs/resources-information-approveddrugs/fda-approves-givosiran-acute-hepatic-porphyria
- Fire A et al (1998), ’Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans’, Nature, 391(6669), 806-811
- Hammond S M et al (2000), ’An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells’, Nature, 404(6775), 293-296 16. Saurabh S et al (2014), ’RNA interference: concept to reality in crop improvement’, Planta, 239, 543-564

Hansjoerg Streicher is senior scientific advisor at Biosynth. He studied organic chemistry at the University of Konstanz, Germany, with a focus on carbohydrates, glycoscience and synthesis. After a period as a visiting scientist at the Weizmann Institute of Science, Israel, he became a lecturer in Chemistry and Chemical Biology at the University of Sussex, UK, where he taught and conducted research for eight years. Hans later moved into consultancy, providing expertise in synthetic chemistry and intellectual property. His work supports project teams in glycoscience and organic synthesis, where he continues to contribute to technical marketing and research initiatives.

Adrian Hery Barranco, marketing and business development officer at Biosynth, holds a PhD in chemistry from the Max Planck Institute for Coal Research (MPI für Kohlenforschung), Germany, where he specialised in advanced molecular design and catalysis. Following a postdoctoral fellowship at the University of Oxford, UK, Adrian joined Biosynth. Here, he leverages his deep expertise in organic chemistry, chemical synthesis and molecular innovation to drive strategic growth. With a strong foundation in substrate chemistry, bioconjugation and drug delivery, Adrian combines scientific insight with commercial acumen, translating complex chemical technologies into market-ready solutions. He plays a critical role in market analysis, strategic planning and partnership development, building strong relationships with academic and industry leaders to accelerate innovation and commercial success.