Process Analytical Technology
A quality boost from mass spectrometry for fermentation processes
Thermo Fisher Scientific discusses the role of Process Analytical Technology in addressing the process challenges concerning fermentation and cell culture in biotechnology and enhancing quality and speed production
Daniel Merriman at Thermo Fisher Scientific
Fermentation and cell culture play central roles in the biopharmaceutical and medical biotechnology industries, and the technologies chosen for crucial process monitoring and quality control (QC) can have a significant bearing on product integrity and yield. For example, these sensitive processes require sterility to be maintained throughout production, but traditional testing methods necessitate the physical removal of samples from the reaction vessel, risking contamination.
This issue – and a variety of other process challenges – can easily be addressed by monitoring inlet and off-gases in real time with process mass spectrometry (MS), allowing the continuous assessment of metabolic activity without the risk of contamination. This article discusses the advantages of employing MS for gas analysis in fermentation processes, highlighting how this approach not only safeguards the sterility of the environment, but also boosts productivity and ensures high quality across the board for biotechnology companies.
The power of PAT
Process Analytical Technology (PAT) uses continuous monitoring and real-time measurements to gain a better understanding of how processes work. The data generated can then be used to refine process control and improve outcomes. Within the biopharmaceutical industry, these outcomes may include ensuring product quality, reducing processing times or accelerating the development for new products. PAT is a multi-step process, beginning with understanding the critical quality attributes (CQAs) for the process that needs to be maintained/improved, then identifying which critical process parameters (CPPs) impact those CQAs and, eventually, continuous monitoring and control of CPPs to improve process outcomes through the selection of appropriate tools. Fermenters and bioreactors are ideal candidates for a PAT approach. These indispensable biotechnology tools serve as controlled environments for the cultivation of microorganisms and cells essential for various applications. Because they are using living organisms, it is vital to regulate environmental parameters to maintain the health and productivity of the cultures and avoid the creation of unwanted by-products. Monitoring of CPPs – such as pH, dissolved oxygen, dissolved carbon dioxide and off-gas composition – can allow real-time process control and response to any unwanted changes in the vessel in order to achieve a high quality, consistent product.
Real-time gas analysis
Careful regulation of various inlet gases – including oxygen, carbon dioxide and nitrogen – helps to create an optimal environment for the growth and metabolism of cells by providing oxygen, aiding the control of pH and temperature and promoting efficient mixing within the bioreactor.
Fermentation also generates off-gases or outlet gases, such as carbon dioxide and other metabolic by-products. Proper gas flow management to remove these off-gases – while minimising any potential loss of valuable volatile compounds – is essential, as accumulation can inhibit cell growth and product formation.
Gas composition analysis plays a crucial role in the monitoring and control of both inlet and off-gases, offering valuable insights into growth kinetics and substrate consumption, as well as determining the optimal point to halt the process for maximum yield. Online monitoring of inlet and outlet gases (see Figure 1) can be used to determine respiratory parameters that provide information about the health of the culture, including:
• oxygen uptake rate (OUR) – the rate at which cells consume oxygen
• carbon dioxide evolution rate (CER) – the production rate of carbon dioxide
• respiratory quotient (RQ) – the CER/OUR ratio.
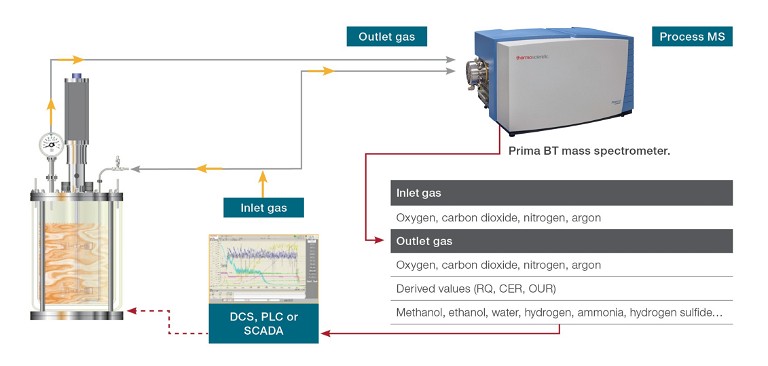
Figure 1: Gas analysis of the fermentation process in a laboratory set-up
Knowing the RQ is essential to understand the health of the culture, indicating both the metabolic efficiency and the type of nutrients being consumed. Precise evaluation of the concentrations of a bioreactor’s inlet and outlet gases – including volatile gases – provides an ideal approach to accurately track a culture’s growth kinetics and substrate consumption in a non-invasive manner, without compromising the sterility of the environment. This data helps to optimise feed times and the start of induction, as well as to determine the ideal time to stop fermentation for maximum viable cell mass. Real-time gas analysis also provides opportunities to identify contamination prior to inoculation, as well as to detect unwanted by-products and the onset of poisoning.
These factors improve overall manufacturing efficiency, reduce over-processing and waste, and contribute to higher biopharmaceutical yields and profits.
Process mass spectrometry for PAT
The choice of gas analysis technology will have a tremendous effect on the success of fermentation process monitoring, as minute changes in carbon dioxide and oxygen concentrations are common, especially at critical time points, such as during the lag phase. Process mass spectrometers have the precision, sensitivity and speed necessary to keep up with these minor variations in gas composition. MS is a relatively simple and non-invasive way to analyse the gases, because it does not require sample collection or the use of sensors that are placed inside sterile areas, helping to prevent contamination. MS platforms also offer more flexibility than alternative gas analysers, because their analytical methods are primarily defined in the software, allowing the analysis of a wide range of sample streams with different compositions. Furthermore, MS is often quicker, more accurate and more versatile than other techniques, and the instruments are self-calibrating with very low maintenance requirements, reducing downtime and allowing continuous use.
Choosing the right tools
Most mass spectrometry workflows consist of the same three key steps – ionisation, mass separation and detection (see Figure 2). There are two types of MS instrument that can be used for gas monitoring in fermentation applications – quadrupole MS and magnetic sector MS. Although the first option is slightly faster and may require lower capital expenditure, it is vital to balance speed with accuracy, as slight gas concentration changes can be significant. This makes magnetic sector MS a superior choice for fermentation gas analysis, as it offers high resolution, accurate mass measurement and precise isotope identification. As a result, magnetic sector MS has been successfully by many of the world’s leading biotechnology and pharmaceutical companies.
Magnetic sector MS uses hot filament electron ionisation to produce positively charged ions from the sample gas.
This is followed by mass separation in a variable magnetic field, where the positively charged ions are accelerated with high energy into a scanning magnetic sector and deflected according to their mass and charge. These deflected ions then enter the detector, where the signal generated by the ions is measured, with the number of ions from each gas molecule being directly proportional to the signal generated at the detector.
This technique offers numerous advantages over quadrupole MS – including higher linearity, accuracy and precision. It also uses a high ion acceleration voltage to produce high energy ions, reducing its susceptibility to scattering by contamination from residual molecules in the vacuum system.
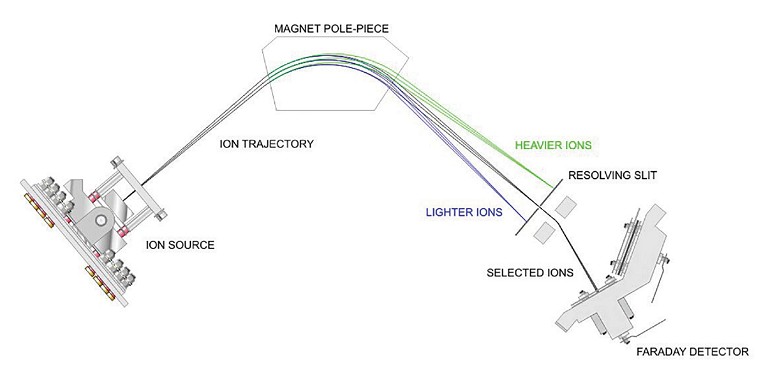
Figure 2: Schematic of magnetic sector MS
This enables the analyser to achieve excellent stability and operate continuously for long periods. Another benefit of magnetic sector MS is that instruments are less influenced by surface charging effects – due to imperfect electrode surfaces – which can result in misalignment or drift in the mass axis. With magnetic sector MS, the signal intensity at any specific mass position appears as a flat-topped peak, removing the need to measure exactly the middle of the peak. This makes the system intrinsically fault tolerant and allows magnetic sector MS systems to have long intervals between calibrations, making them extremely beneficial for prolonged fermentation processes, which can last days or even weeks.
Rapid multi-stream sampling
Monitoring several bioreactors simultaneously requires a fast and reliable way to switch between streams. Common approaches often involve solenoid valve manifolds or rotary valves, but these options have significant drawbacks; the first has too much dead volume, while the second suffers from poor reliability. More advanced inlet systems offer significant benefits over these traditional approaches, including high sampling speeds and reliability for extended periods of continuous use. Stream settling times can be configured by the user to best suit the application, and while digital sample flow recording for each stream can be used to trigger alerts if the sample flows drop, enhancing overall process security.
PAT plays a pivotal role in the biopharmaceutical sector by addressing regulatory standards and ensuring efficient process control. The purpose of PAT is to provide valuable insights that can be acted upon to optimise a production process; this approach often includes inline, at-line and online measurements that can evaluate key parameters in real time and feed the data into a control system. Ultimately, use of PAT will significantly shorten production and development cycles and minimise the risk of poor product quality. The use of real-time gas analysis is particularly beneficial for monitoring fermentation and cell culture processes, thanks to advancements in innovative and user-friendly MS devices. These systems present a straightforward and non-invasive method for analysing gases within the workflow, eliminating the need for sample collection or the placement of sensors in the sterile fermentation environment, thereby minimising the risk of contamination. Notably, magnetic sector MS provides crucial data – such as the OUR, CER and RQ – empowering operators to make well-informed decisions regarding feed times and when to stop fermentation. This strategic approach not only significantly enhances yields and profits, but also ensures the production of more consistently high quality biotherapeutic products.

Daniel Merriman has worked in the field of process analytics for the chemical and biotechnology industries since 1988. He has held many positions across technical, sales and marketing, and now supports the strategic marketing group within the Analytical Instruments Group, EPM of Thermo Fisher Scientific.