
Manufacturing
Innovations in pharmaceutical Manufacturing Including Capsuling, Tabletting, Additive Manufacturing, Biomanufacturing Operations
How is semantic layering technology addressing significant efficiency gaps, creating the infrastructure for more efficient manufacturing operations?
Process innovations provide opportunities to reduce environmental impact while improving efficiency and product quality
How is X-ray CT being used to ensure quality in tabletting?

In biologics development, clone selection is not just a technical milestone, it’s a strategic decision with far-reaching clinical and commercial implications. How can thoughtful clone selection – integrated with developability and manufacturability assessments – accelerate speed to clinic while safeguarding long-term success?

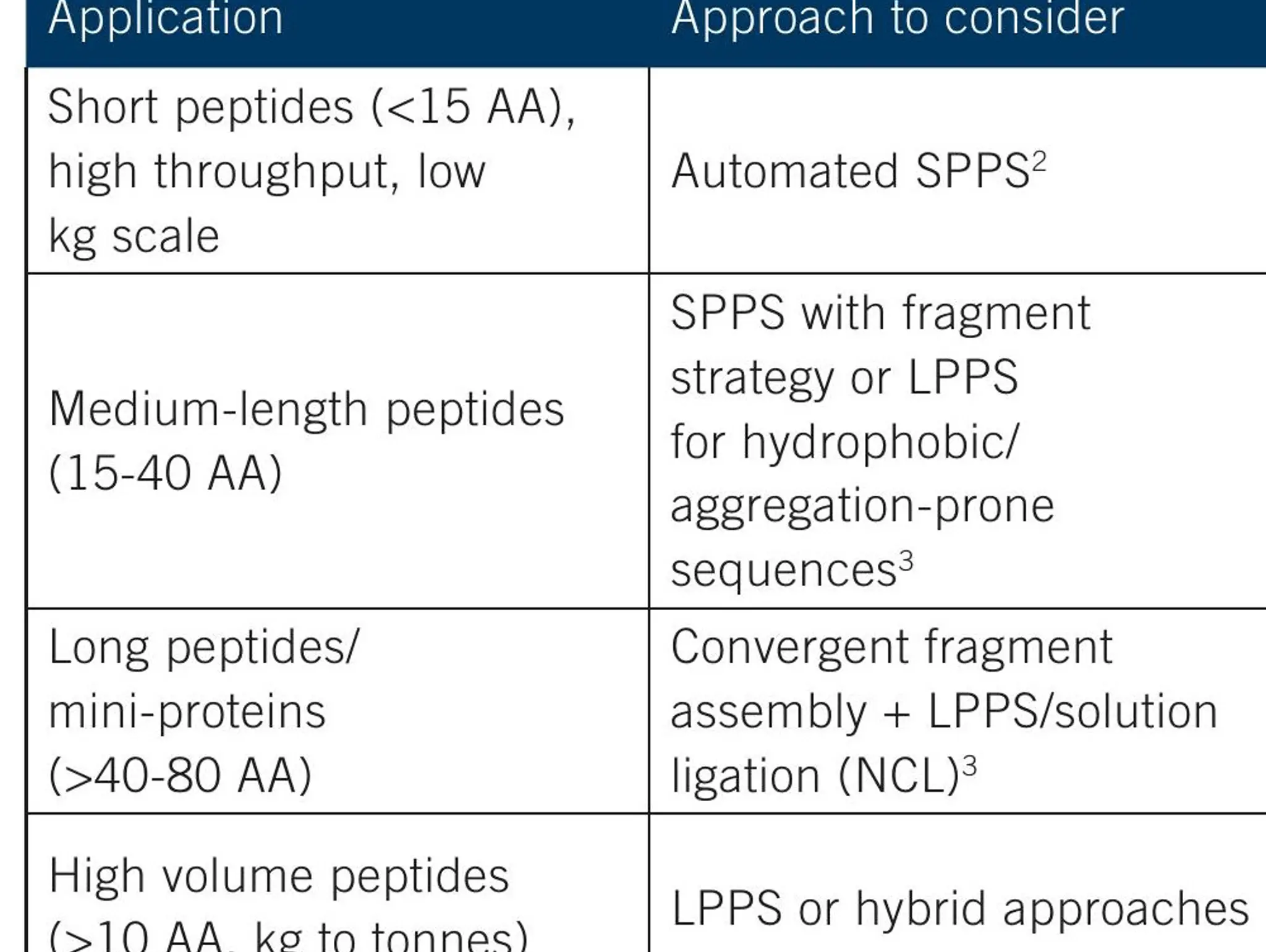
Biocatalysis is enhancing complex molecule process development. By merging the power of enzymes with chemical synthesis, scientists and manufacturers are addressing long-standing challenges in stereoselectivity, route complexity and sustainability

SPOTLIGHT: How CARBOGEN AMCIS supports complex molecules and challenging formulations from early development to commercialisation

What are eight compelling reasons why manufacturers should prioritise the use of advanced contamination detection systems in their operations?

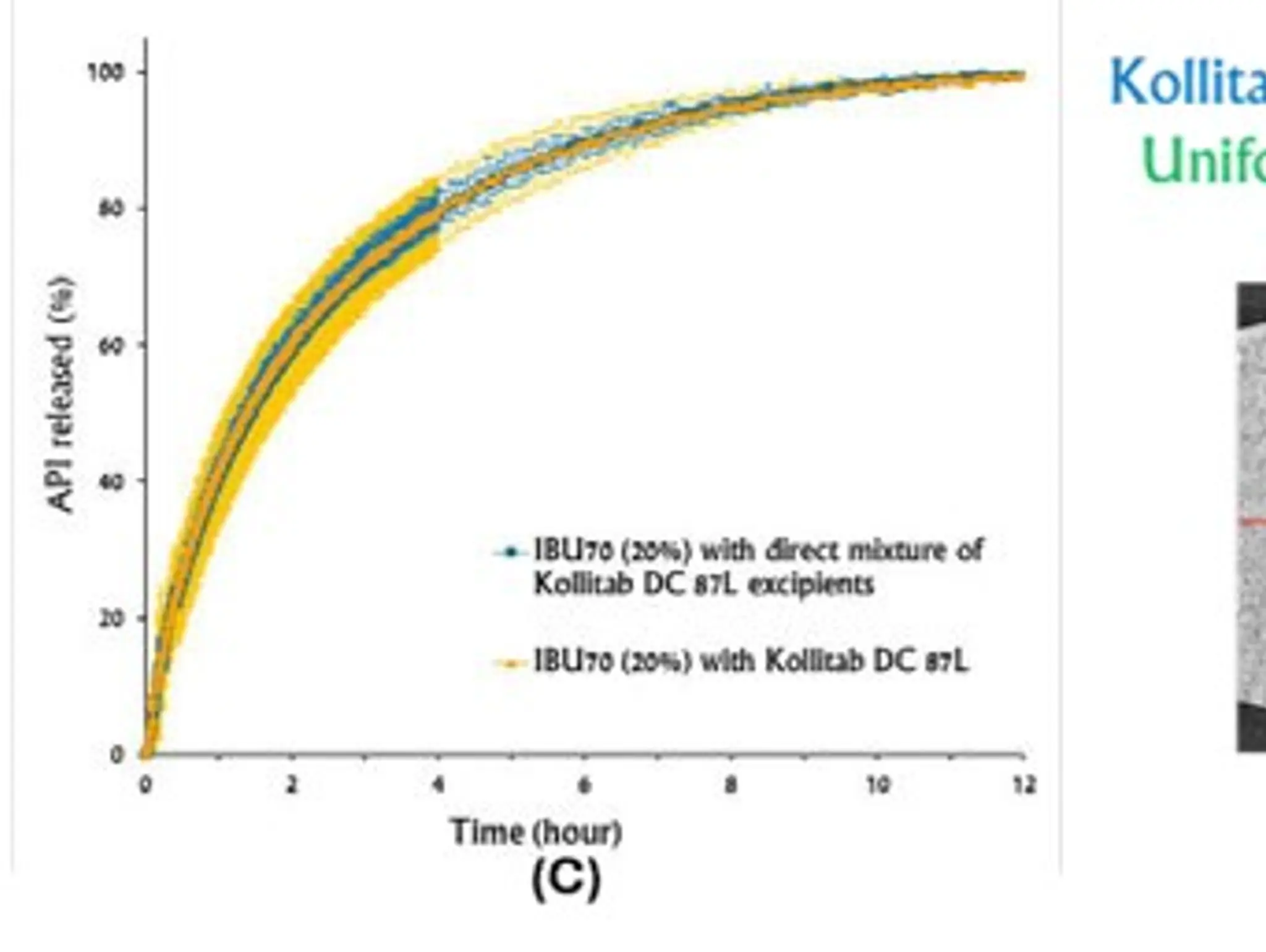
SPOTLIGHT: Bill Supplee at Natoli Engineering discusses the best ways to ensure tablet density uniformity in pharma products

Sustainability is top of the agenda for many pharmaceutical manufacturing companies. Which role do technology providers and their equipment play in reducing CO2 emissions

Why is the use of synthetic DNA a better choice for emerging genetic medicines?

What are some innovative techniques used for manufacturing gene therapies at a commercial scale?

Tablet formulation development often uses small R&D tablet presses to optimise both the composition of the formulation and manufacturing process parameters. These types of machines have a wide variety of capabilities and may be either single station or small rotaries.