Manufacturing: Topical Medicines
Advances in In-Line Rheology Measurement of Topical Pharmaceuticals
Reinventing a section of pharmaceuticals that has traditionally stayed the same for decades is not easy, but new developments in topical medicines are showing us the pathway to innovation
Ken Primrose and Thomas Machin at Stream Sensing
Topical pharmaceuticals, used to treat conditions such as rashes, skin irritation, fungal infections, muscle pain etc., are manufactured as lotions, creams, foams, solutions, suspensions, or gels. Products can be either a water in oil (w/o) or oil in water (o/w) emulsion, containing waxes, emollients, and lubricants dispersed in an oil phase, emulsifying, stabilising, and thickening agents in a water phase, plus preservatives. The active pharmaceutical ingredients (APIs) may be dispersed in either phase, or added when the emulsion has been formed and cooled.
A typical manufacturing process includes:
- Preparation of the oil phase, where flake/powder ingredients are dispersed into mineral oil or silicone oil
- Hydration of the aqueous phase, where emulsifiers, thickeners, and stabilisers are dispersed into water and may be heated to accelerate hydration
- Emulsion, where the two phases are blended under vigorous agitation to form the emulsion
- Dispersion of the API, where the API is efficiently dispersed to maximise yield and product effectiveness
To abide by the strict regulation of pharmaceutical products, it is critical that the end product is of a consistent texture or viscosity so that it can be applied correctly, and deliver the right quantity of API effectively to the patient. The association between clinical performance and a product’s microstructure is dependent on critical manufacturing process parameters, such as rheological characteristics. Due to the complexity of the production process, measuring rheology throughout is key to ensuring the required consistency, stability, and quality in topical pharmaceuticals.

Quality by Design
Quality by Design (QbD), the 21st Century systematic and risk-based approach to pharmaceutical product development and manufacturing, requires a good understanding of how raw materials and process parameters influence the final quality profile of a product. Although QbD has been adopted by many since it was introduced by the FDA in 2002, its full implementation is still limited, especially in the case of semisolid complex formulation development (1).
In these types of products, developers first define the quality target product profile (QTPP), a list of quality attributes (QAs) that are required in the final product. These might include elements such as dosage form, administration route, particle or globule size, rheological behaviour, drug concentration, homogeneity and uniformity, pH, in vitro drug release and permeation, microbial limits, etc. The QAs can be affected by both the ingredients used and the manufacturing procedure parameters. Therefore, critical material attributes and critical process parameters should be specified (2).
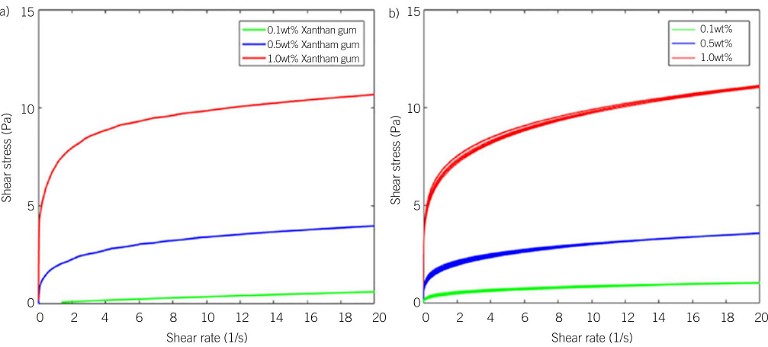
Figure 1: Comparison for xantham gum of electrical resistance rheometry (ERR) and rotational rheometry: a) Shear stress vs shear rate plot generated from a rotational rheometer; b) Shear stress vs shear rate plot generated using an ERR
QAs that relate to rheology in topical pharmaceuticals include: viscosity as a function of shear stress and shear rate, G’ (storage modulus), G” (loss modulus), LVR region (linear viscoelastic region), and yield stress (2). Non-Newtonian materials, such as topical semisolid products, do not flow unless they have reached a critical stress level called ‘yield stress’. The yield stress point also correlates well with sensorial properties of topical formulations, such as spreadability and ease of application (3).
Rheological characteristics can have an impact on drug release from the formulation, skin penetration, and skin retention of the topical dosage forms, as well as the formulation’s stability, physical appearance, and performance throughout shelf-life (4, 5, 6). Many processing factors can affect the final rheological characteristics, including mixing speed and duration, plus any minor changes in the composition of ingredients such as the thickening agents. So, it is critical that rheology is monitored and controlled throughout manufacturing.
Variations in rheology can have a major impact on the end product and even result in product failures. The consequences of such a failure are significant and include: changes in skin retention of the formulation and drug penetration through the skin, reduced patient acceptability/compliance, and impact on sensorial attributes of the product (2).
Rheometry Testing
Conventional rheometry testing typically takes place offline with sampling required from the process stream. This can take approximately 10 minutes (depending on the number of measurement points required), not including time to obtain the sample, send to the QC lab, and conduct internal checks. Once analysed, if the product does not meet the required specifications, it often as to be scrapped or reworked, resulting in waste being generated, or the use of excess energy.
Case Study: Xanthan gum
Xanthan gum is a polysaccharide produced by a biotechnological fermentation process and is used in many pharmaceutical applications as a suspending agent, an emulsion stabiliser, and a foam enhancer in semi-solid, liquid, and topical formulations.
Machin et al compared the rheological parameters of xanthan gum powder in water obtained from ERR with offline measurements. They found that the acquired ERR rheological parameters were seen to mimic those obtained from a conventional rotational rheometer, possessing an accuracy of 97.4% and 98.5% for k (consistency index) and n (power index), respectively.
“ERR, a novel technology, has been shown to provide accurate measurements of rheology”
The rheological properties from off-line measurements are often also considered as directly applicable to real process flows. However, this method only provides a retrospective characterisation of a fluid sample and the measurement does not account for any changes to the sample when extracted from process (e.g., cooling down, gelling/settling).
As in situ measurements are conducted within the flow environment, they deliver a direct, real-time rheology measurement, which provides essential, accurate information to plant managers and QC professionals about the product as it is being processed. In the case of topical pharmaceuticals, any changes in QAs identified can be swiftly remedied via the addition of rheology modifying materials or changes to mixing speed and duration, for example. The ability of manufacturers to make these adjustments quickly is critical in them ensuring compliant and successful end products.
Introducing Electrical Resistance Rheometry
Machin et al developed a novel, in-pipe, tomographic measurement capable of obtaining real-time rheological information of process fluids within pipe flows, ERR (7).
ERR enables the characterisation of a fluid’s rheological properties within a pipe under steady state, incompressible, laminar flow conditions. The wide-ranging applicability and simple implementation of micro-electrical tomography sensors to industrial processes make it an ideal platform for the development of an in-line rheometer. This approach is an extremely robust, non-invasive technique, which can interrogate complex process fluids. ERR can combine significant engineering quality and process control concepts of rheology and mixing to form a powerful characterisation tool.
Conclusion
ERR, a novel technology, has been shown to provide accurate measurements of rheology – akey functional product quality in the production line in real time, and could result in huge savings, shorter lead times, and improved quality control for manufacturers of complex liquids, such as topical pharmaceuticals. Ensuring the rheology is correct in these products is key to product quality and effectiveness, and the adoption of this technology could overcome some of the key constraints of traditional offline methods.
References

Ken Primrose is CEO of Stream Sensing, with over a decade of experience. He took a small incubator company that was responsible for commercialising technologies developed by the University of Manchester, UK, and grew it into Industrial Tomography Systems: the world-leader in tomographybased process visualisation tools. In 2020 Ken led the spin out of Stream Sensing Ltd, which makes real-time, in line rheology instrumentation.

Thomas Machin is the Senior Technology Officer at Stream Sensing. He studied chemical engineering at the University of Birmingham, UK, where he went on to gain a doctorate in 2020 and develop the technology that Stream Sensing is now helping the industry to use.