Streamlining Processes at CDMOs
Integrated development strategies for drug product optimisation
Drug product optimisation, also known as reformulation, is a development step required by all drugs en route to market. This process takes a long time, carries a considerable cost and places significant emphasis on the predictive power of laboratory and preclinical assessments. What are some alternative pathways available to development teams?
John McDermott at Quotient Sciences
All drugs require changes in formulation when en route to market. The exercise of drug product development can be triggered for any number of reasons. Common examples include transitioning from an early development formulation to one suitable for chronic administration in patients, seeking to promote exposure in response to physiochemical and biopharmaceutic properties of the drug, as well as developing modified release strategies to improve treatment regimes.
Typically, reformulation starts in the laboratory, where the drug product development team works to identify formulation prototypes that address the needs of the molecule. These formulations are screened in dissolution and preclinical studies − where successful drug products are scaled up at a contract development and manufacturing organisation (CDMO) − to manufacture clinical supplies. A clinical study is then conducted to determine product performance in humans. This process requires plenty of times, significant cost and labour. It is a suboptimal working model necessitated by the multiple disciplines required to develop, make and test new formulations in humans. Integrating formulation development, good manufacturing practice (GMP) and clinical testing activities dramatically streamlines the reformulation process by enabling the manufacture and dosing of test formulations in days rather than weeks or months.
What sort of reformulation might be required and when?
Early development
In early development, relatively simple and fit-for-purpose formulations are frequently employed to expedite entry into clinical development. The formulation strategy at this stage is designed to provide high dose flexibility and allow cautious steps to be taken in evaluating the safety and tolerability of the drug in a first-in-human (FIH) study. Once FIH is completed, the product may need to be reformulated to ensure the dosage units administered are safe and suitable for proof-of-concept (POC) patient studies.
Many development groups are seeking to bridge pharmacokinetic (PK) studies in order to characterise the in vivo performance of the new formulation. This is due to the increase in compounds that are exhibiting challenging physicochemical and biopharmaceutical properties in today’s drug pipelines, meaning that development teams must scale up the GMP manufacturing process and generate several months of stability data for a product with several months of shelf life.1 These processes incur a considerable time and cost burden to the development budget.
Independent assessments state that this process, from formulation development through to completion of the bridging PK study, can take between 12-18 months. This is time well invested if the new formulation is proven to be successful, as the development team is assured that the drug has the best possible chance of achieving physiological exposures to be efficacious during POC. However, if it is not a successful profile, then the development team must repeat the formulation development cycle at the expense of time and financial burden.
Tackling reformulation challenges
Taking an integrated approach to drug product optimisation projects in the early development stage by leveraging translational pharmaceutics (TP), (Figure 1) enables full drug substance, drug product and clinical testing activity integration.2 Performing and consolidating these tasks under one organisation and a single programme manager works to streamline processes. Manufacturing, release and clinical dosing cycle times can be reduced from months to days. This significantly alters the transition from formulation development to bridging the PK data set. This seamless integration of activities allows for closely aligned workflows that efficiently conserve active pharmaceutical ingredient (API) consumption, reduce overall development risk and costs and fast-track molecules from candidate development to FIH and onward to GMP drug products for POC studies.
“Traditional non-integrated development models cannot provide a fast enough response to learn from data emerging from the clinic to quickly manufacture new formulations”
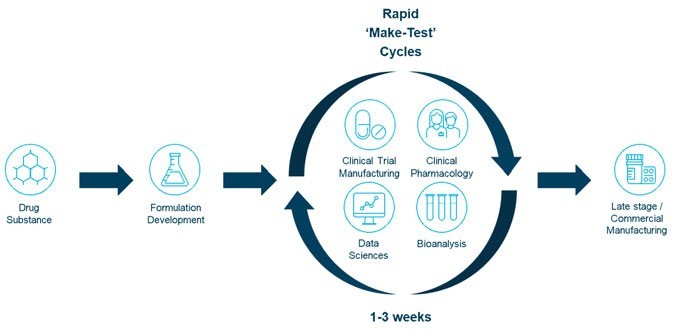
Figure 1: Translational pharmaceutics
Late Development
In late-stage development, a complete reformulation of an API is usually only justified if there are significant issues with the formulation’s performance in manufacturing (eg, in scale-up), prior to use (eg, stability) or in use (eg, performance). However, an exception to this might be where there have been significant changes to the target product profile (TPP), which can occur when there’s a requirement to transition to a sustained release formulation. In this situation, there could be a switch to a different product format, route of delivery and/or required use of alternative formulation technology. While the changes made to the formulation could be significant, learnings from the previous formulation development programme are invaluable in informing development and key performance targets (eg, PK parameter targets) to give the new development programme a head start. Depending on the formulation technology employed, critical-to-performance formulation variables may be identified, such as the levels of functional excipients. As the effective formulation required to achieve the desired performance may not be known, formulators find ways to optimise the drug product composition in dissolution and preclinical experiments – although these are frequently found to be poor predictors of performance in human subjects.3,4 Another way to perform the same optimisation is to use clinical data. However, traditional non-integrated development models cannot provide a fast enough response to learn from data emerging from the clinic to quickly manufacture new formulations and return for further dosing.
Integrated approaches such as TP can enable this strategy by accelerating the drug product manufacture, release and dosing cycle time, to make learning and responding to emerging data possible within a two- to three-week cycle time. This can be further enhanced by employing a ‘formulation design space’ (Figure 2).
The use of the term ‘design space’ in the context of pharmaceutical drug development has become synonymous with quality by design (QbD) principles. This is a defined formulation and manufacturing ‘safe space’ where in vivo product performance will not be affected.5 Regulators have expectations that the industry will therefore develop and demonstrate an understanding of their drug products and processes to justify control strategies and specifications accordingly.
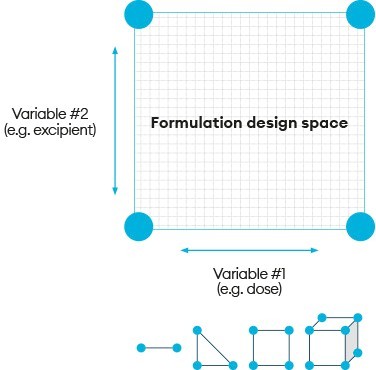
Figure 2: Formulation design space
Combining formulation design space with the ability to manufacture products in real time allows for immediate critical-to-performance formulation adjustments to be made in response to emerging clinical data. Critically, this ‘make, test, modify, make, test’ cycle can be executed during the FIH clinical study with complete freedom, provided the compositions selected stay within the pre-approved formulation design space. This, therefore, enables enhanced precision in the selection of formulation compositions to make and dose for eliciting the desired response. Studies can be run with one, two or more component variables, creating a multidimensional formulation design space to achieve successful drug delivery even when the formulation risks or complexity are high.
Formulation design space, when employed within a TP programme, inverts this concept to achieve a range of formulation compositions where it is fully expected that in vivo product performance will vary as compositions are changed. Regulatory approval of this formulation design space is achieved by including representative batch release data and appropriate (short-term) stability data from the space of the extreme composition in submissions, to provide assurance over the product quality of any discrete composition within the defined range.
Summary
In summary, drug products may require reformulation and optimisation at any stage in the drug development life cycle. Successful drug product optimisation is dependent on many factors and can be difficult to achieve within traditional industry silos as the time and costs required to develop and manufacture new formulations are significant.
This approach has driven risk acceptance in the productiveness of in vitro, in silico and preclinical models. Development of a unique TP platform, however, has changed this by integrating formulation development, GMP product manufacture and clinical testing activities under a single organisation and single clinical protocol. These same benefits that were once restricted to laboratory scientists and experiments, enhance the speed for clinical testing and maximise within-study flexibility in the responding of arising human clinical data. Experience has now demonstrated the broad applicability of this innovative approach for the optimisation of drug products. In particular, how the embodiment of a formulation design space can allow the tuning of quantitative formulation compositions in the clinical setting to reduce risks and improve the likelihood of downstream success.
Application of a TP can therefore address key industry challenges associated with accelerating the drug product optimisation process to get new medicines to patients faster.
References
- Hauss D (2007) Oral lipid-based formulations, Advanced Drug Delivery Reviews 59, 557-676.
- Scholes P et al (2009) Translational Pharmaceuticals – Interactive Drug Development to Enable Rapid Optimisation of Drug Products in Early Development. Presented at: American Association of Pharmaceutical Sciences (AAPS) Conference, Los Angeles, CA, US, 8-12 November.
- Grass G M et al (2001) Effect of diverse datasets on the predictive capability of ADME models in drug discovery’ Drug Discovery Today 6.12 (Suppl.) S54-S61.
- Musther H et al (2014) Animal versus human oral drug bioavailability: do they correlate?, Eur J Pharm Sci 57, 280-291.
- Visit: ema.erupoa.eu/en/ich-q8-r2-pharmaceutical-development-scientific-guideline

John McDermott, VP, scientific consulting at Quotient Sciences has over 20 years industry experience in roles in pharmaceutical sciences. He has been central to the development of Quotient Sciences’ translational pharmaceutics platform, which integrates formulation development with clinical manufacturing, regulatory support and clinical testing, to help achieve flexible and efficient first-in-human studies and fast development. In addition, John has a significant body of experience in scintigraphy, imaging studies for oral and inhaled dosage forms, including the development and validation of radiolabelling methods.