Gene Therapies
Non-viral gene therapies: GETting growth-factor-based healing right in orthopaedics
The development and use of novel gene therapy approaches is increasing. With the advent of innovative technologies using improved tropism and safer viral-based systems, and nanotechnology advancing non-viral delivery of nucleic acid vectors, there will be more gene-based products entering the market in near future
Anandkumar Nandakumar and James Dixon at TherageniX
Gene therapy is an alternative method to direct the introduction of therapeutic proteins into the body (Table 1). Delivery of the gene encoding the protein of interest has several benefits, which allow for the increased control of the platform that therapy can be tailored specifically for such as:
- The amplification of one gene, generating large numbers of mRNAs and in turn producing large numbers of protein, allows low mass delivery to generate much more therapeutic product
- The tissue targeted, itself, generates the therapeutic protein, meaning it is correctly folded, post-translationally modified and its function is as close to its endogenous counterpart as possible
- That same tissue is the depot of therapeutic protein production, so the effect is localised and less likely to have off-target effects
- The therapy can be engineered to last longer than the stability of recombinant proteins delivered directly. Moreover, the expression can be tailored to super- or physiological-levels or -durations, making dosing much easier to control
- In certain systems, gene therapy can be cheaper and easier to engineer than expression and purification of a recombinant pure active protein
- The plug-and-play nature of non-viral gene therapy allows genes to be swapped or even multiple genes, with different expression kinetics, to be co-delivered.
Improving intracellular delivery using non-viral vectors
The use of non-viral vectors for gene transfer is considered safer and simpler than the use of viral vectors, but with a low efficiency gene delivery. Polymers, lipids and peptides have all been developed as platforms to encapsulate gene therapies. These vectors effectively diffuse and mediate the uptake and functional cell localisation to allow for the transfection and subsequent expression of delivered nucleic acids.
One of these approaches uses cell-penetrating peptides (CPP), which were initially based on cationic TAT HIV peptides to mediate compaction of nucleic acids into complexes for cellular uptake. To improve intracellular delivery and gene expression efficacy using non-viral vectors, while also lowering the dose required, a multidomain CPP platform has been developed.1 This glycosaminoglycan (GAG)-binding enhanced transduction (GET) system (Figure 1), comprises a GAG-binding peptide sequence (termed P21 or fibroblast growth factor (FGF)2B) to stimulate cell membrane interaction, fused with nucleic acid compaction and endosomal escaping sequences (such as LK15) and a CPP (such as octa-arginine; 8R). It can be several orders of magnitude more effective at nucleic acid internalisation than TAT-based systems.
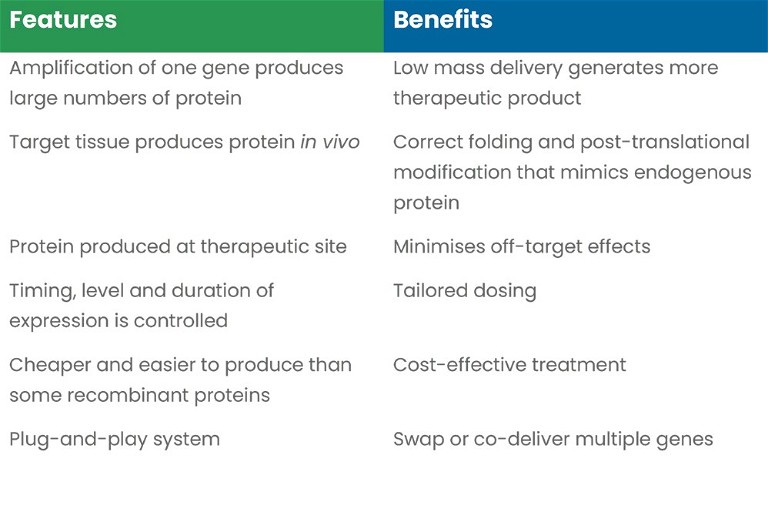
Table 1: Overview of the benefits of non-viral gene therapy

Figure 1: Glycosaminoglycan (GAG)-binding enhanced transduction (GET) system. FGF2B, GAG-binding peptide sequence: fibroblast growth factor 2B; LK15, nucleic acid compaction and endosomal escaping sequences; 8R, cell-penetrating peptide: octa-arginine
The GET peptide relies on GAG-binding to enhance intracellular delivery efficiency by concentrating the formulation onto the cell membrane, promoting the effectiveness of CPP-mediated internalisation. It is capable of efficiently delivering nucleic acids, enzymes, hormones and antibodies to a number of different cell types for several applications, including gene editing/monogenic disease gene correction, regenerative medicine and vaccinology, at low doses.
The use of GET complexed, with plasmid DNA as a gene therapy, generates therapeutic nanoparticles for orthopaedic regenerative enhancement (Figure 2). Transgenically augmented expression of potent osteogenic and angiogenic factors: bone morphogenic protein 2 (BMP-2) and vascular endothelial growth factor (VEGF), respectively improved the close of critical size defects in the treatment of bone in vivo.1
Autologous grafting for bone regeneration
Gene therapy holds much promise as a new treatment option for large bone defects and has the potential to make a significant impact on patients’ lives. Musculoskeletal conditions affect approximately 1.71 billion people worldwide, being the highest contributor to the global need for rehabilitation. Some of the main contributors to musculoskeletal conditions are those affecting bones, such as osteoporosis, osteopenia, and associated fragility and traumatic fractures, which affects 440 million people globally.2
Bone regeneration naturally occurs in most cases, but in traumas such as non-union or malunions, and diseases such as tumours that cause bone defects and avascular necrosis, bones may not heal independently. This then requires surgery and bone grafting is often employed to induce bone regeneration. Bone grafting is used in orthopaedic, oncologic and dental procedures, with autologous grafting seen as the gold-standard treatment. Worldwide, there are two million bone-grafting procedures performed annually. Bone regeneration therapy currently has limitations that cause societal economic burden and reduced quality of life for patients and there is a need for regenerative therapies that are affordable, accessible and have better clinical outcomes for those affected.3,4
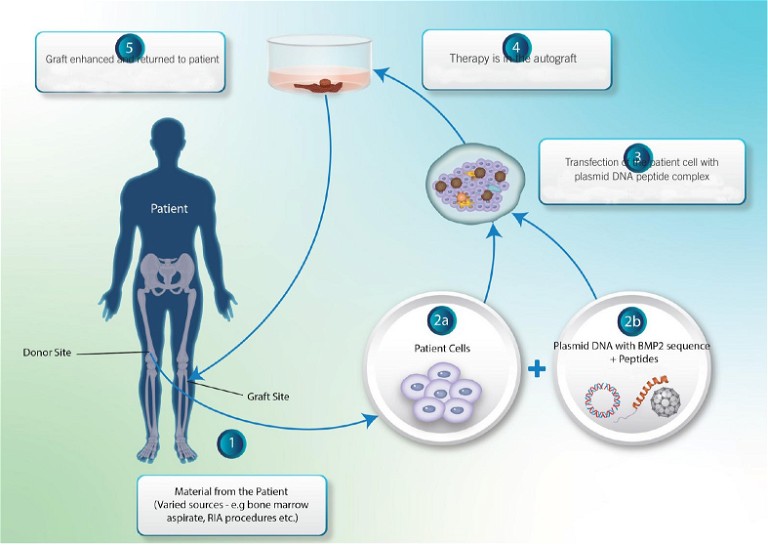
Figure 2: GET complexed with plasmid DNA generates therapeutic nanoparticles for orthopaedic regenerative enhancement. BMP, bone morphogenic protein; GET, glycosaminoglycan (GAG)-binding enhanced transduction; RIA, reamer-irrigator-aspirator
Autologous grafting uses bone harvested from the patient’s own body (eg, iliac crest and intramedullary canal of long bones) as a filler to augment healing.5 These types of materials have been shown as osteogenic, osteoinductive and osteoconductive, and contain viable bone precursor cells. Since autologous grafts are freshly isolated from the patient, they contain several endogenous growth factors: BMP-2, BMP-7, FGF, VEGF, platelet-derived growth factors (PDGFs) and insulin growth factor-1. Autologous grafts remove the risk of rejection and disease-transmission; however, these must be harvested from another surgical site, bringing risks such as additional pain, increased infection and donor-site long-term morbidity.6,7 Autologous grafts can also resorb too quickly before new bone growth is complete and integrated.8
Some procedures may use bone marrow aspirate, which is minimally invasive compared to reamer-recovered material and has a high number of mesenchymal stem cells to aid fracture healing.9-11 Other less common treatment options have included autologous grafts from cancellous, cortical and vascularised cortical bone. Also extracts from blood, termed platelet-rich plasma, have been used as additives with other strategies. Allogenic grafts include cancellous, cortical allografts and demineralised bone matrices processed after recovery. Furthermore, synthetic bone grafts such as calcium phosphate ceramics, tricalcium phosphate and hydroxyapatite, among others have been used for bone regeneration.
Re-evaluating recombinant proteins
In order to augment healing, graft materials are often combined with growth factors and bioactive molecules. More recently, those incorporating recombinant human (rh) growth factors, such as BMP and PDGFs, have been approved by the US Food and Drug Administration (FDA). BMPs are a group of transforming growth factor-beta proteins that play a crucial role in bone remodelling and repair.12 In recent years, rhBMP proteins have been introduced into clinical settings for a variety of indications, including long-bone fracture repair. Local administration of BMP-2 and BMP-7 in tibial non-union fractures resulted in increased healing rates.13,14 Although successful in generating mineralised bone, safety issues arose from the increased and off-label use of these molecules; these clinical issues were significant including inflammatory complications, benign seromas, cervical spine swelling and risk of tumour formation. As BMP can upregulate tumour cell proliferation and invasion, and ectopic bone formation due to the active protein being used in vast excess to propose longer responses and ectopic activity outside of the implant site, such direct and high-level dosing of these molecules has fallen from favour.15,16
Orthopaedic regenerative gene therapies
In orthopaedic regenerative gene therapies, BMP gene therapy using the GET system provides a sustained secretion of the protein in a localised manner by specific cells that enhance the therapeutic effect. Furthermore, co-delivery of VEGF synergistically enhances angiogenesis and overall osteogenesis.
Transgene expression can be engineered to mimic the natural process of bone healing, without the need for high doses of BMP delivered during surgery. Studies have shown that non-viral BMP gene therapy can induce the secretion of pico- to nanogram levels of protein, compared to the micrograms needed in recombinant-protein-based commercial products. In addition, only a short term of BMP-2 or -7 expression is required to obtain bone regeneration and fracture healing in animal models.17,18 Therefore, gene therapy allows a therapeutic strategy that could, in the future, be more cost-effective, efficacious and safe with optimal clinical outcomes for those with bone defects that will otherwise not heal, causing significant pain, loss of function and lower quality of life. Given the benefits of non-viral gene therapy systems like GET, companies are developing gene therapy products for orthopaedic applications.
Gene therapies: present and future
There are over 30 FDA-approved cell and gene therapy products as of June this year.19 Almost two-thirds of these are cell therapy products that focus on different types of cancers and the use of cord blood cells after myeloablative treatments. The ten gene therapy products approved use different viral vectors. While the initial products have focused primarily on viral vectors, perhaps due to the higher efficiency of gene delivery, non-viral vectors hold great promise and potential. New approaches like the GET technology, along with other non-viral delivery modes, will encourage the exploration of these methods, leading to safer therapies in the future.
Besides the use of novel non-viral vectors for delivery, the administration and formulation aspects of gene therapies are also highly exciting areas for innovation. While most currently approved products require a single dose, there are now products that can be administered more than once, in intervals ranging from once a week to once every few months. The possibility to administer repeated doses and control the release of therapeutics in a simple way could also help to advance non-viral vector-based approaches by offsetting their potentially lower efficiency. A powdered gene therapy could also help to reduce the cost of treatment and improve access. Compared to current liquid formulations, such an approach would offer higher stability, allowing gene therapies to be transported and stored more easily.
This in turn could lead to equity in access to groundbreaking therapies, particularly in high-temperature zones, where expensive cooling equipment may be unavailable.
With the first set of products approved and a vast array of innovative approaches in the pipeline, gene therapy is expected to grow in the coming years. Researchers are leveraging technologies such as gene sequencing and profiling, and synthetic biology to advance not only the delivery and efficacy but also the manufacture, storage and transportation of these therapies. In bringing novel therapies to market, we hope to address unmet medical needs − in orthopaedics and beyond − and ultimately, improve the lives of patients.
References
- Raftery RM et al (2019), Highly versatile cell-penetrating peptide loaded scaffold for efficient and localised gene delivery to multiple cell types: From development to application in tissue engineering, Biomaterials Sep 216: 119277.
- Visit: who.int/news-room/fact-sheets/detail/musculoskeletal-conditions.
- Gillman CE et al (2021), FDA-approved bone grafts and bone grafts substitute devices in bone regeneration, Mater Sci Eng C Mater Biol Appl Nov 130: 112466.
- Bez M et al (2020), BMP gene delivery for skeletal tissue regeneration, Bone Aug 137: 115449.
- Dimitriou R et al (2011), Complications following autologous bone graft harvesting from the iliac crest and using RIA: a systematic review, Injury 42 Suppl2: S3–15.
- Pape HC et al (2010), Autologous bone graft: properties and techniques, J Orthop Trauma 4 Suppl 1: S36–40.
- Kloss FR et al (2017), Comparison of allogenic and autogenous bone grafts for augmentation of alveolar ridge defects–A 12-month retrospective radiographic evaluation, Clin Oral Implants Res Nov 29(11): pp1163-1175.
- Giannoudis PV et al (2005), Bone substitutes: an update, Injury 36(3): S20-S27.
- Roberts TT et al (2012), Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing, Organogenesis 8(4): pp114-124.
- Baldwin P et al (2019), Autograft, allograft, and bone graft substitutes: Clinical evidence and indications for use in the setting of orthopaedic trauma surgery, J Orthop Trauma 33(4): pp203–213.
- Khan SN et al (2009), The biology of bone grafting, J Am Acad Orthop Surg 13(1): pp77–86.
- Dumont CE et al (2009), Reconstruction of large diaphyseal defects of the femur and the tibia with autologous bone, Eur J Trauma Emerg Surg 35(1): pp17–25.
- Risniemi J et al (2007), RhBMP-7 accelerates the healing in distal tibial fractures treated by external fixation, J Bone Joint Surgery Br Feb 89(2): pp265-272.
- Govender S et al (2022), Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures, J Bone Joint Surg Am Dec 84(12): pp2123-2134.
- James AW et al (2016), A review of the clinical side effects of bone morphogenetic protein-2, Tissue Eng Part B Rev 22(4): pp284–297.
- Carragee EJ et al (2011), A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned, Spine J 11(6): pp471–491.
- Bez M et al (2017), In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs, Sci Transl Med May 17 9(390): eaal3128.
- Wang RN et al (2014) ,Bone morphogenetic protein (BMP) signalling in development and human diseases, Genes Dis Sep 1(1): pp87-105.
- Visit: fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products.

Anandkumar Nandakumar is CEO at TherageniX, a company pioneering the use of gene therapy for tissue regeneration. Anand has close to 20 years’ experience in the life sciences industry across medtech, biotech and pharma. After completing his PhD in bone tissue engineering, he worked for a medtech start-up leading the development of novel polymer-based scaffolds for cardiovascular applications, culminating in an FIH study in young children. After that, Anand spent time in an MNC focusing on vaccine development and later at a scale-up leading the operational readiness of the company’s first CAR T-cell therapy facility in the Netherlands.

James Dixon is an associate professor of the School of Pharmacy and NIHR Nottingham Biomedical Research Centre at the University of Nottingham, UK. James is the inventor of the GET gene delivery technology used by TherageniX for enhanced regenerative medicine. He is an expert in developing nanoparticle technologies for gene augmentation and provides the expertise to apply his technology to vaccines, cancer therapy, regenerative medicine and gene correction/editing platform.