Manufacturing: formulation development
The key to formulation development is in the details

During the tablet manufacturing process, tooling and press manufacturers are often faced with ongoing challenges, but how can a thorough approach to this reduce manufacturing issues, costs and downtime?
Robert Sedlock at Natoli Scientific
During the tablet manufacturing process, tooling and press manufacturers are often faced with ongoing challenges. As a tooling and press manufacturer, you should be able to expect support from your partnering vendor during all aspects of the compression process. These services should include press operator training, maintenance/calibration services, quick delivery of parts, tooling/tablet design and powder formulation support.
If any compression issues occur, these services act as a support tool to help you overcome manufacturing challenges. However, if there is a change in formulation, a process following Scale-Up and Post Approval Changes (SUPAC) guidelines must occur before moving forward. Time-consuming and often frustrating, the SUPAC process is guaranteed to halt manufacturing and should be avoided.
This article will review the importance of the formulation development process and how a thorough approach can reduce manufacturing issues, costs and downtime. The case studies provided in this article were performed at the Natoli Institute for Industrial Pharmacy Research & Development – Arnold and Marie Schwartz College of Pharmacy and Health Sciences on the Brooklyn, NY campus of Long Island University, US.
For both formulators and research professionals, developing a tablet formulation that is both robust and able to be scaled into manufacturing without issue can be a challenge. Tasked with this, formulators and research professionals use their understanding of the science side of powder compaction to effectively communicate with scale-up and manufacturing groups – increasing return on investment and reducing time to achieve a marketed product. Single-station tablet presses offer many advantages during early development, such as requiring a limited amount of material to characterise potential formulations. With only a few milligrams of material, the die filling process can be performed manually. Characterising just the API can also be performed on these machines allowing the scientist to select the appropriate excipients based off the results of the compacted API.
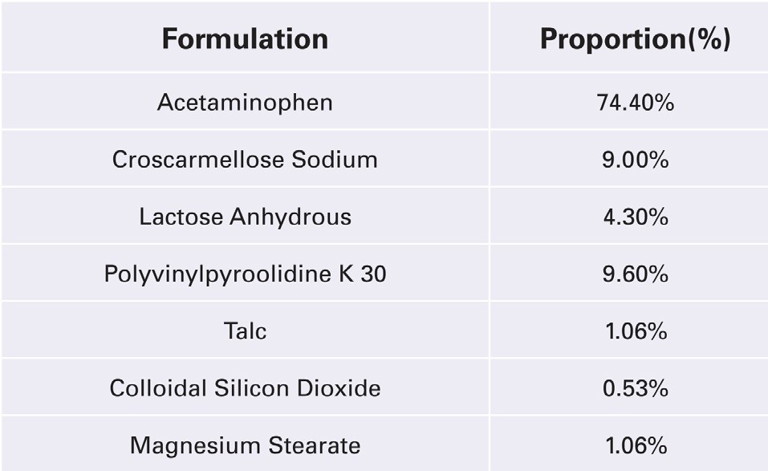
Figure 1 is a tabletability profile performed on the NP-RD10A single-station tablet press. This is an example of evaluating a high drug load directly compressible APAP formulation (DB) versus a wet granulated APAP formulation (WG).
Instead of collecting the data using compression force (kN), there is an advantage to normalising the punch tip face area and utilising compaction pressure. With any new formulation the required compression force is based on the material properties and tablet size. Performing a compaction study from 50MPa–300MPa covers the normal ranges for compressing pharmaceutical tablets of all sizes.
Furthermore, the tablet tensile strength is a normalisation of the tablet geometry. Target hardness or a breaking force value (kilopond or Newtons) cannot be determined until further testing is performed. Target tensile strength of 1–2MPa are representative values for a robust tablet that will withstand handling, friability and coating operation.
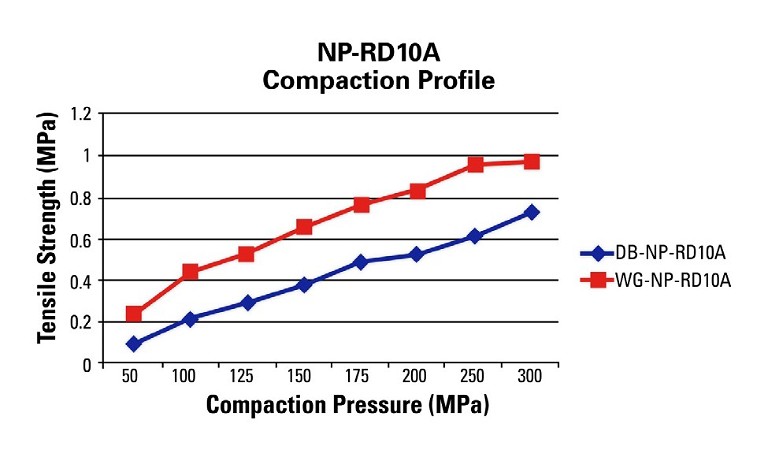
Figure 1: Natoli tablet press, NP-RD10A, Natoli BLP-16
The profile clearly shows that the wet granulation blend yields stronger tablets than the directly compressible blend throughout the compaction pressure ranges. Although, there is a cost advantage with directly compressible blends as the equipment investment costs and process times are much lower. The next step in the process is to perform additional studies on a rotary tablet press.
Figure 2 depicts the tabletability profile performed on the BLP 16 rotary tablet press at 20 RPM turret speed for the DB and WG formulations. The BLP16 rotary tablet press is a 16-station B tooled machine that is capable of running at variable turret speeds.
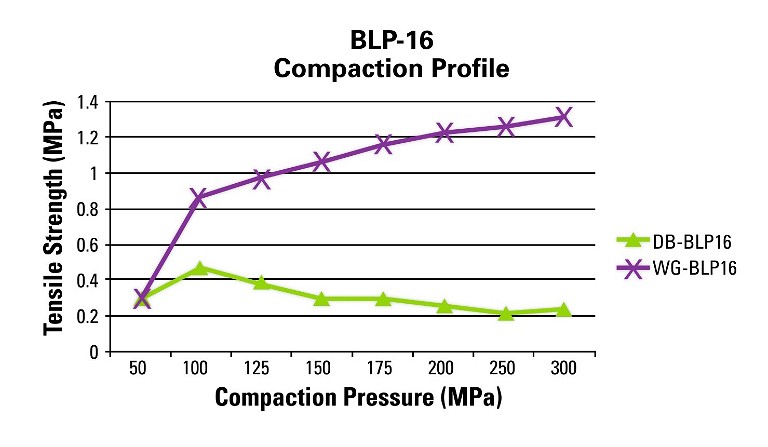
Figure 2: Tabletability profile performed on the BLP 16 rotary tablet press at 20 RPM turret speed
The profile shows different results as compared to the RD10A single-station tablet press. This data is representative of what can be expected at the manufacturing level since the manufacturing machines utilise the rotary press design with upper and lower compression rollers.
In this case, the upper and lower punches are moving horizontally across the rollers and vertically to compress the powder whereas the single-station machine only moves vertically for non-eccentric designs. This difference changes the dynamics of the compression event and has an impact on the final tablet attributes as shown in this example (Figure 3).
The WG formulation run on the BLP16 yields robust tablets above 1MPa tensile strength at a reasonable compaction pressure where the DB formulation exhibits capping above 100MPa of compaction pressure. It is clear that the WG blend is the choice formulation, and that the BD blend will fail on a manufacturing rotary press. Profile A is an example of good tabletability, whereas Profile B is an example of when tabletability is acceptable at lower compaction pressures but deteriorates at higher pressures and Profile C is an example of poor tabletability.
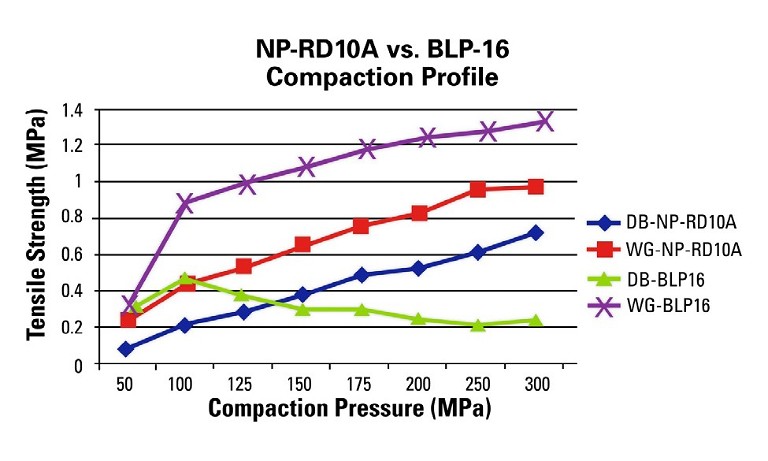
Figure 3: Single-station vs. rotary press tabletability profile depicts the compaction profiles for the two different machines
Many factors can impact the tabletability curve including the powder deformation characteristics, particle size, shape, moisture content, amount of powder fines. Every product behaves differently on a tablet press, even varying when run on a different day. Variation is due to changes in the properties of the raw materials, API and excipients from batch to batch.
As shown by the data in Figure 4, it is crucial that your product has a robust tabletability profile. Profile B is an example of where your tablet attributes might be acceptable – at research or scale-up levels – but fails at the manufacturing level. Operating too close to the peak of the curve or on the descending side offers no flexibility to the press operator when issues are found and with the many variables as described above this is crucial.
The use of pre-compression can improve your tabletability profile as it de-aerates and consolidates the material before the main compression event; but it is wise to save this tool as a backup when issues arise at the manufacturing level. Developing a robust formulation without pre-compression will allow the manufacturing press operators to make press adjustments to solve these issues.
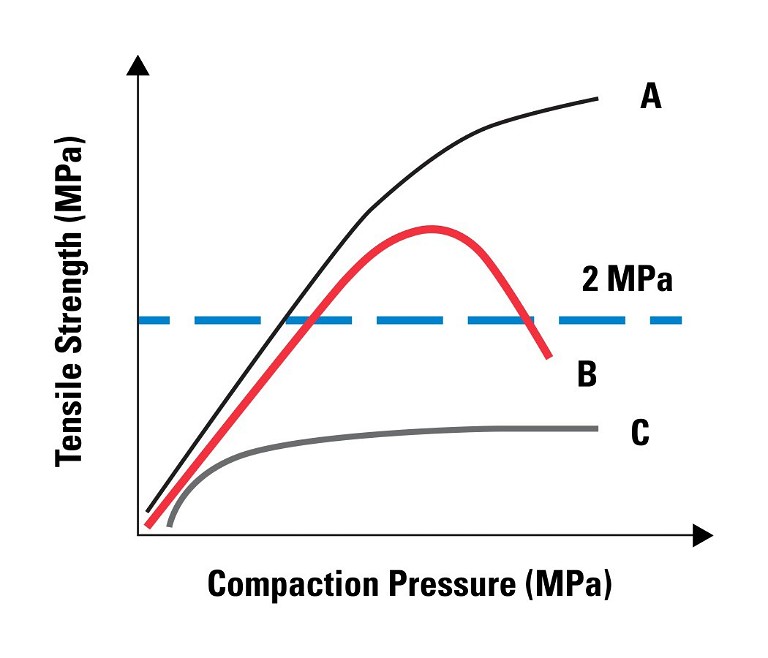
Figure 4: Examples of different tabletability profiles
Another study that is valuable in the development process is a strain rate study, where the compaction pressure is held constant and, in this case, we chose 150MPa since tablets were robust at this level, the turret speed was incrementally increased and tablet attributes were evaluated.
Instead of evaluating tablet strengths at different turret speeds it is a more scalable approach to normalise for the turret pitch circle diameter, punch head flat diameter and evaluate the tablet strengths as a function of dwell time, which is the time under maximum compression force or when the punches are no longer moving vertically. Another important parameter to consider for press scalability is the punch vertical velocity or loading rate and the decompression event. In Figure 4 we can observe that this WG formulation is not strain rate sensitive at dwell times as low as 13 milliseconds.
Ultimately the product will be produced on a high-speed manufacturing press, and the dwell times can be calculated and further studies can be performed at the research level. As most research presses are designed with smaller diameter turrets, reaching similar velocities as the manufacturing machines cannot be achieved so your tooling head profile can be designed to simulate similar dwell times.
The methods above are ways to evaluate your potential formulation's tabletability and scalability; now let's discuss how the ingredients that make up the formulation impact the final tablet quality. Apart from the tablet’s active ingredient, other essential components include diluents, binders, disintegrants, lubricants, glidants, colouring agents and more. This next section will describe the importance of powdered lubrication (ie, magnesium stearate) and the negative effects of excessive amounts.
Lubricants are essential for successful tablet manufacture. They provide a means to reduce the friction at the interface between a tablet’s surface and the die wall during the tablet ejection event. High ejection forces and excessive friction can cause premature wear to the lower punch heads and press cam tracks, visual striations on the tablet belly band surface and lamination. Lubricants can also improve flowability as it reduces the inter-particle friction.
With the many benefits of adding lubricant, there are negative effects when the amounts are excessive and not optimised. An excessive amount of lubricant can cause tablet strength issues and increased disintegration and dissolution times, and with the tablet press paddle feeder this issue can be exacerbated when feeder speeds are too high. The feeders could potentially over-blend the formulation causing the lubricant to coat more particles inhibiting the bonding process during compression.
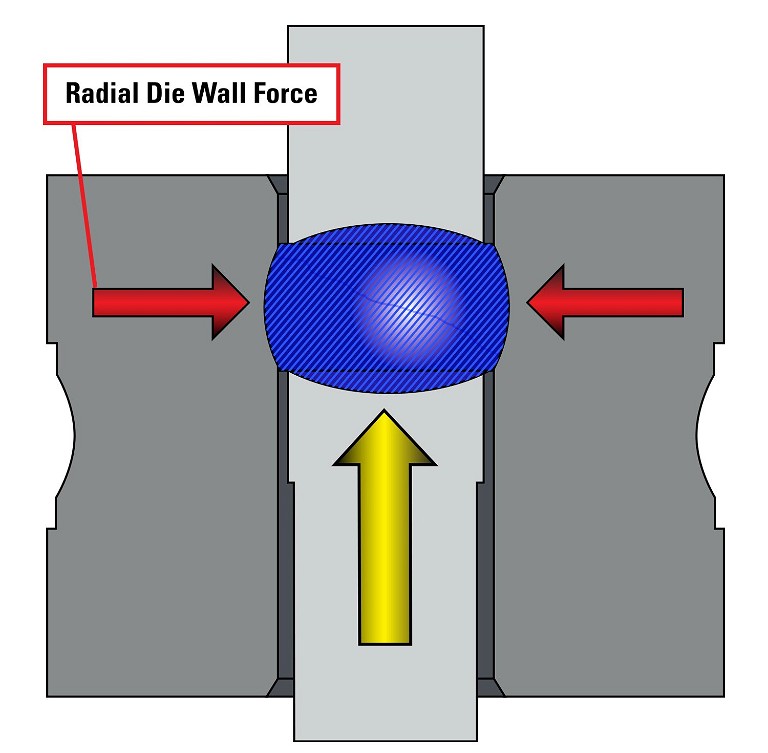
Radial Die Wall Force illustration
As lubricants are hydrophobic, it will prevent the penetration of fluids, hence the disintegration and dissolution issues. Furthermore, when the tablet press is not set up properly and the selected fill cam is not appropriate for the dosing cam setting, the excessive scrape-off material is recycled back into the feeder for further mixing.
Performing a lubricant study in the development process with varying amounts of lubricant will identify the optimum level. During a compaction profile study as described earlier, the ejection force can be recorded as the applied compression force is incrementally increased. Since the ejection force is the product of the residual radial die force and the coefficient of friction between the tablet belly band and die wall, the ejection force should increase with increased compression. If the ejection force does not increase with increased compression, the friction is reduced, indicating the lubricant level can be decreased.
Furthermore, a properly designed ejection transducer is required to accurately measure the ejection event. As most tablet presses adjust the lower roller position to change compression force and tablet thickness the lower punch head will contact the ejection cam at a different location depending on the setting.
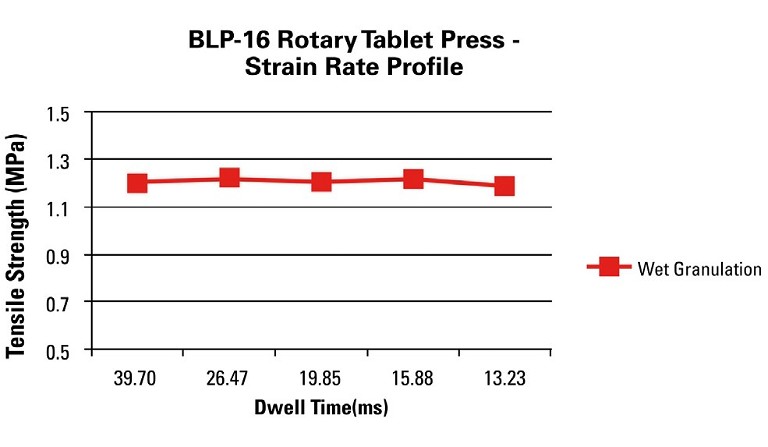
Figure 5: A full travel transducer design
A full travel transducer design (Figure 5) is insensitive to punch position whereas a button load cell design is only accurate at one contact point. Figure 5 is an illustration of the ejection event. This example adds another variable where the die has an excessive wear ring resulting in higher ejection forces and tablet quality issues.
Conclusion
With the many challenges in the pharmaceutical manufacturing world today, tooling and press manufacturers are your key partners in the support of delivering a quality product. Tablet manufacturing issues arise from many variables including operator training, calibration and maintenance of equipment and the formulation that is compressed.
It is crucial in the development process to optimise the formulation and evaluate tablet robustness from tabletability and scalability studies. This will help minimise the challenges faced at the manufacturing level and will allow the press operators to make press adjustments when many variables arise unexpectedly.
Natoli Engineering Company offers hands-on training opportunities for tablet manufacturing processes. For more information, call a Natoli representative at +1 636-926-8900 today.

A Natoli Engineering product