22 articles from this collection:

IPT Winter 2022 Cover

ICON
ICON. Clear Focus. Better Outcomes.

IPT Winter 2022 - Contents

EDITORIAL DESK
As another year draws to a close, it’s the perfect time to reflect on the past while moving unwaveringly into the future.
Cell Phenotyping and Functional Measurements Using Flow Cytometry
IPT recently spoke with Xiaobo Wang at Agilent about what’s been driving demand for flow cytometry and where the technology might be heading

Raman Analysers Take Centre Stage to Streamline Pharmaceutical Quality Control
How are handheld Raman devices and process analysers helping to streamline Quality Control (QC) procedures and enable continuous manufacturing?

Pharma Role

Five Factors Driving Cell and Gene Therapy Developers Towards Gammaretrovirus
What has led to the rising trend for Cell & Gene Therapy (CGT) developers to choose retroviral vectors over other potential vectors, and what are the many drivers behind this selection process?

Collaboration and Centralisation: The Key to Successful Drug Development
IPT talks to Dr Fiona McLaughlin and Neil Bell of Avacta Therapeutics about the need for cohesion when researching and developing a new drug between life science companies, academia, and medical practitioners

PM GROUP

The Pioneering Tech Speeding Up Drug Development
IPT talks to Johannes Stanta at Celerion about how improvements in molecular and cellular capabilities are aiding Good Laboratory Practice/Good Clinical Practice (GLP/GCP) standards

DEL, AI, and ML: The Digital Future of Research Tools
IPT talks to Noor Shaker at X-Chem about the adoption of AI and ML into life sciences and how pre-trained algorithms can aid research

Turning an Ocean of Data Into Actionable Wisdom To Support Optimal Site Identification
Advancements in AI can help the pharmaceutical industry identify the best sites for their clinical trials through the harnessing of Big Data

The Burden of Proof – Implementing Blockchain in Life Sciences
Blockchain technology is fast becoming a mainstay in pharma companies. What is the draw for these companies to use it, and how can it help improve data collection and protection in the future?

International Clinical Researcher of the Year 2023
International Clinical Researcher of the Year 2023 is now open for entry

Digitalisation – A Giant Step Towards Global Access to Medicine
How will the mass integration of digital technologies into the pharmaceutical industry benefit the most underserved countries?
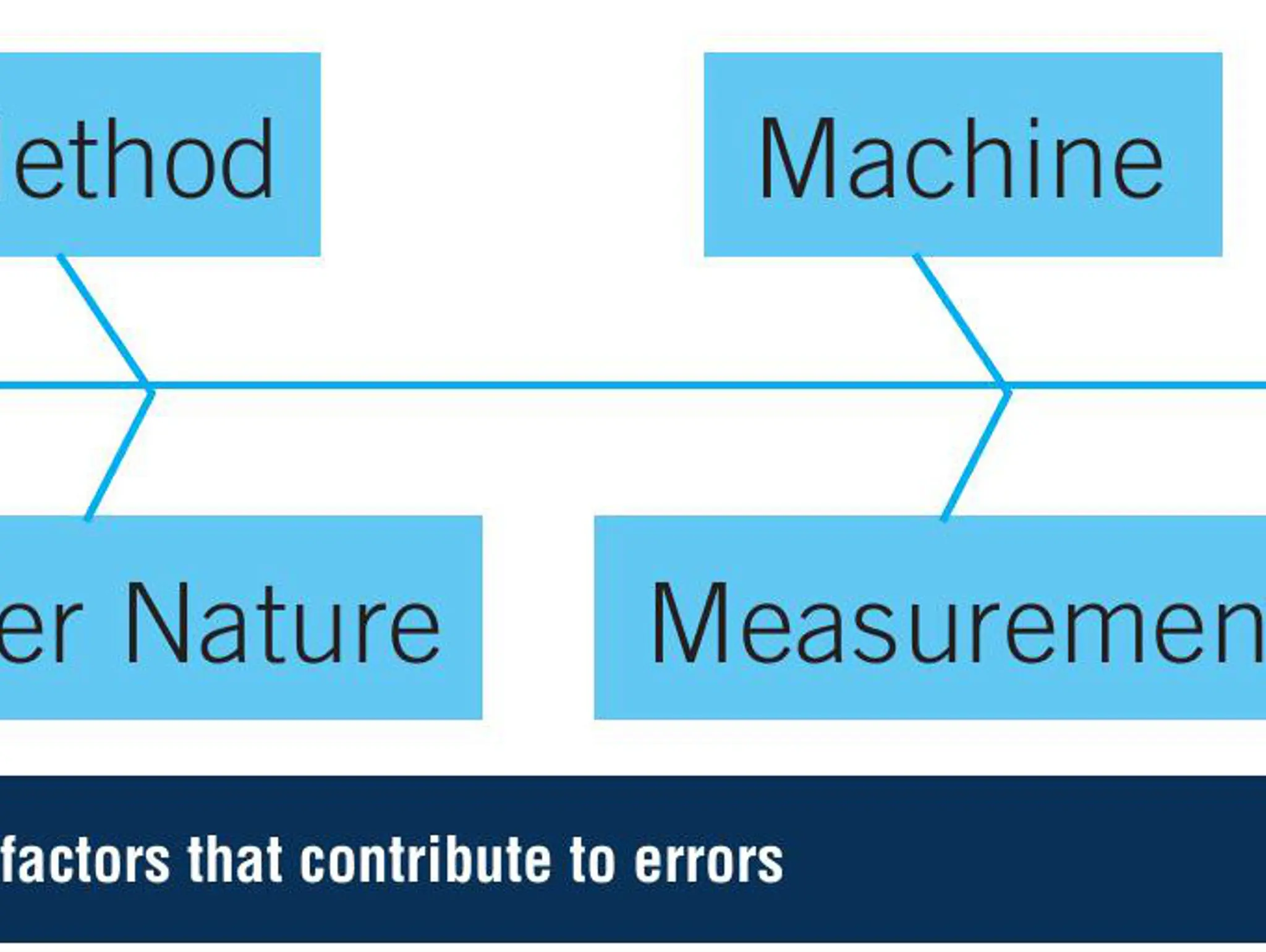
Error Mitigation in Pharmaceutical Quality Assurance and Control
Ensuring pharma products are manufactured in a safe and controlled way is a priority. How can the industry ensure that this happens?
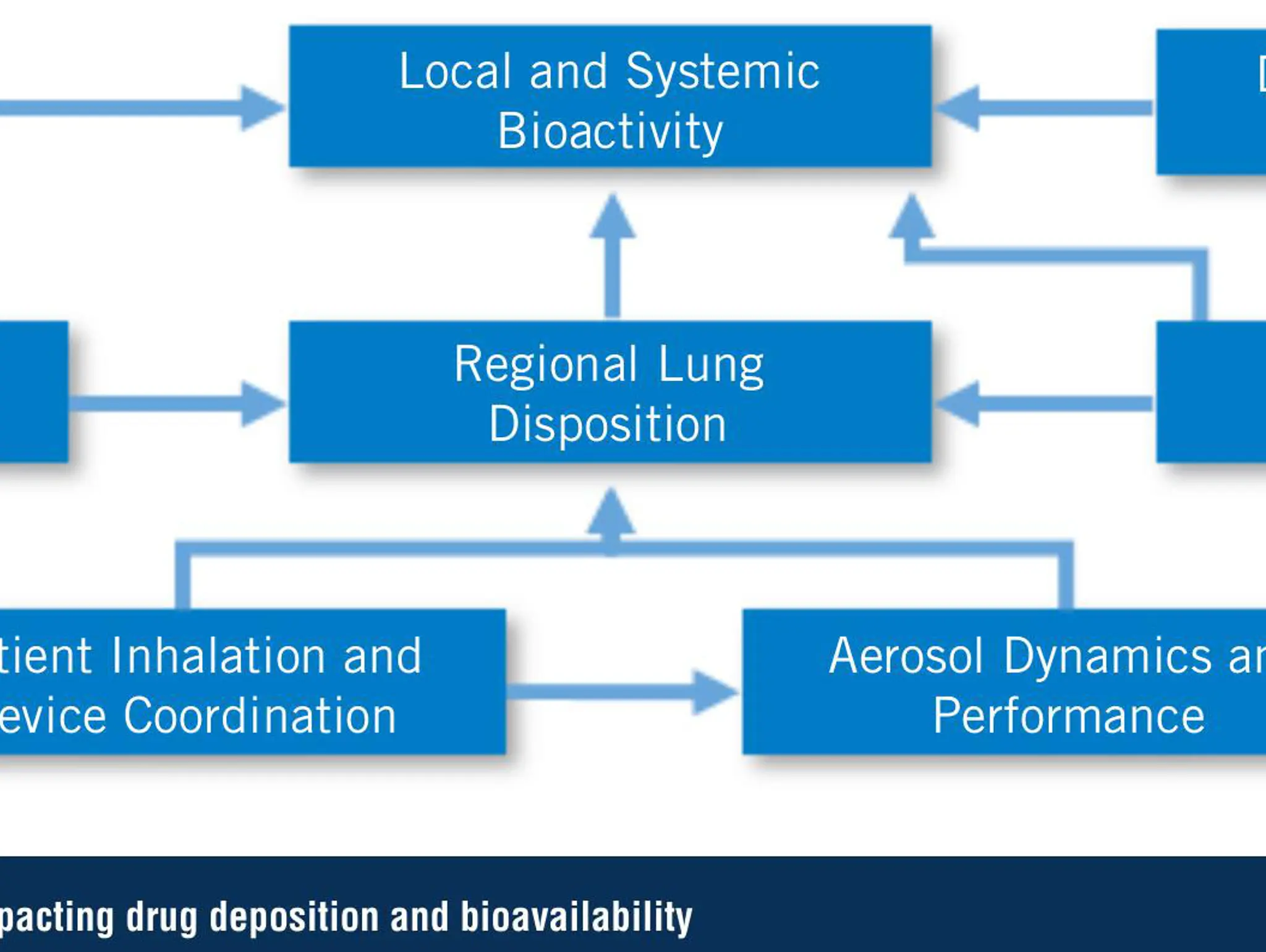
An Alternative Regulatory Pathway for Generic Orally Inhaled Drug Products
The in vitro-in silico ‘alternative’ bioequivalence pathway for generic inhaled drug products, and how to factor in patient variability to successfully replace your comparative clinical end-point study

Managing the Complex Regulatory Pathways for Bioprinting Breakthroughs
Bioprinted products are already starting to demonstrate life-changing potential, but the path to commercialisation is complex, requiring developers to better understand the factors that impact regulatory classification

EVENTS

SAMEDAN

NATOLI
Natoli - Introducing the NCF-45 automatic encapsulation machine
