Analytics: Microscopy
Advances in Acousto-Optic Multiphoton Laser Scanning Microscopy
The ability to visualise in greater detail and with better accuracy can offer laboratories an array of benefits. What new developments are entering the industry, and how can they overcome obstacles to analysis?
Dénes Pálfi at Femtonics
The intricate interactions of neurons with their surroundings form the basis of the complex network dynamics underlying neuronal computation. Understanding the nature of information processing through these interactions is one of the long-term goals of researchers studying how neuronal activity is linked to specific behaviours. To get closer to this goal, they need to measure neuronal activity in the brain while it is hard at work and actively processing information.
The above-mentioned networks of brain cells are 3D and are connected via fine processes. Visualising such elaborate structures in action, in particular detecting fast events occurring simultaneously at dispersed locations in 3D space (for instance, across spines on a neuronal dendritic tree), has been made possible by current advances in in vivo imaging techniques. In this article, we will focus on a novel technology particularly efficient in performing such challenging measurements: acousto-optic (AO) multiphoton laser scanning microscopy.
Perfecting the Methods of Measuring Brain Activity
Measuring neuronal activity has traditionally been based on electrophysiological recordings performed in vivo or in vitro. However, this technique is not only invasive and laborious, but is also incapable of measuring the activity of hundreds of neurons simultaneously. Calcium imaging, on the other hand, enables us to visualise the activity of large neuron populations with substantially better spatial resolution (1, 2). Calcium imaging using multiphoton laser scanning microscopy is now the method of choice for many neuroscientists, as it allows for the optical accessibility of deep tissues in vivo, with low phototoxicity. With this technique, brain regions spanning over multiple cortical layers can be reached, down to a 1,000µm depth, and recorded in 3D with high spatiotemporal resolution and signal-to-noise ratio (SNR). Multiphoton microscopy has made it possible for neuroscientists to visualise the brain of awake, behaving animals in vivo in 3D, providing deep penetration into live and functioning tissue – from the network scale, to dendrites, and dendritic spines (3).
Behind Acousto-Optic Laser Scanning Microscopy
While laser scanning microscopy was a real breakthrough for neuroscientists, users of optical sectioning by laser scanning microscopy still faced the difficulty of only being able to scan one plane – moving between planes in the Z direction in a slow manner, mechanically. Therefore, several new optical methods have been developed recently for recording 3D structures. 3D AO scanning enables single-point multi-photon excitation, which allows for the whole-field detection of the scattered fluorescence photons required for imaging in the deepest regions of the brain, even whole cortical columns (4). This unique technique increases the measurement speed and signal collection efficiency significantly, compared to classical raster scanning method performed with the same pixel dwell time (5).
The AO technology uses TeO2crystals instead of mechanical scanners, which function as electrically-controlled deflectors of light: they are tuned by sound waves, making it possible to guide the laser beam in all planes, including the Z-plane. These deflectors enable the excitation beam to jump between any points with a 30kHz speed in samples, which means that the AO technology allows for the detection of neuronal activity in 3D volumes of deep brain tissues, with a much higher speed than conventional scanning methods (see Figure 1) (5).
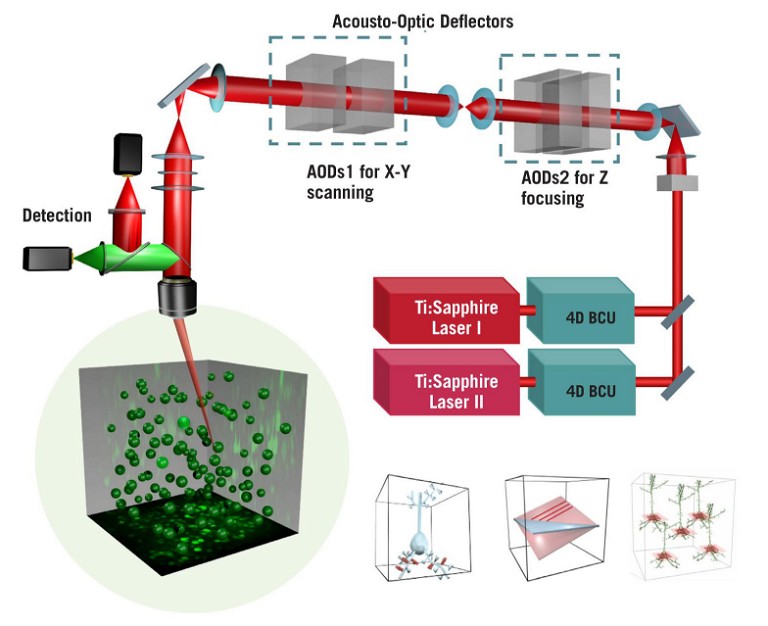
Figure 1: Schematic of the optical light path of the FEMTO3D Atlas acousto-optic two-photon laser scanning microscopeIPT
Efficient Simultaneous All-Optical Access to Multiple ROIs in 3D
State-of-the-art, ultrafast multiphoton imaging platforms, such as the FEMTO3D Atlas AO platform, can select and target regions of interest (ROIs) precisely and rapidly, up to kHz sampling rates, without wasting measurement time on unnecessary background volumes (see Figure 2, page 14). With this technology, a scanning volume of a cubic millimeter can be imaged with high resolution with a manifold improvement in measurement speed and SNR, making the technology suitable for precise multisite activity measurements, especially when the ROIs are sparsely dispersed in the 3D volume.
3D random-access point scanning powered by the AO technology is the foundation of the flexibility of Atlas, making experimental strategies possible that have been out of reach so far due to the limitations of conventional 2D microscopes. In random-access point scanning mode, Atlas can jump across a series of submicron-sized locations, requiring a very short amount of time (30µs) to reposition the focal point to the next point, achieving faster data collection in 3D than traditional plane-by-plane scanning methods. Atlas now implements ‘drift scanning’ modes that recover this short time during ‘jumps’ to extend points into 3D lines. Aligned lines can form freeform 3D surface or volume scan patterns, depending on the structure of the target features. Using drift-based 3D scanning modes allows researchers to record continuously along any intricate structures they are interested in (see Figure 3, page 15) (3).
The ‘Chessboard’ scanning mode of the Atlas can scan up to 300 cell somas dispersed in 3D anywhere in a near cubic millimetre volume by drifting the laser beam, then visualise them in a practical chessboard arrangement. This technique has proven to be immensely useful in recent ambitious studies involving large-scale multiphoton imaging of neuronal network dynamics in the hippocampus in awake mice performing a plenitude of behavioural tasks (6, 7).
High-Speed Arbitrary Frame Scanning With Acousto-Optic Laser Scanning Microscopy
Researchers often need to take volumetric scans to capture an oblique structure in their samples. The FEMTO3D Atlas AO platform accommodates this need with the high-speed arbitrary frame scanning mode, through which cells assembled into a large neuronal network dispersed in a 3D brain volume can be acquired with a frame rate of 40Hz in a 500×500µm field-of-view. Beyond the high speed, the main advantage of this mode is that the selected plane can be rotated to any angle in space, without mechanical constraints. Moreover, to cover more cells in the neuronal network, the scanning plane can be extended into multiple layers, forming a Z-stack perpendicular to the original focal plane. This can be used as an alternative to Bessel beam technology, following the dynamics of neuronal networks in a 3D tissue (8).
Simultaneous Photostimulation and Imaging
Photostimulation – another quite revolutionary technology – allows researchers to quickly modulate and monitor a vast number of neuronal events by light-activating cells in a selective and precise manner. The Dichro extension of the FEMTO3D Atlas platform combines the strengths of the AO technology and optogenetics. A second laser coupled to the system enables users to perform optogenetic stimulation or uncaging simultaneously with calcium imaging, even in different cell populations, by rapidly switching between the two laser lines. Using the 3D random-access excitation method of the FEMTO3D Atlas Dichro, scanning methods of the AO technology are available with flexible parameters for photostimulation. Depending on the selected scanning pattern, researchers can stimulate sparsely distributed cells or dendritic processes in a large volume with high precision (8).
Overcoming Motion Artefacts
Pre-existing 3D anti-motion technologies apply the latest theoretical results implemented in the control software of acousto-optic microscopes to extend sampling points in 3D into a line along any specified direction (3). When many identical lines are precisely aligned, a free-form plane can be scanned in 3D. During an in vivo experiment, the sample almost always exhibits motion artifacts due to vessel pulsation, respiration, or limb movements. Scanning in ribbons or cubes ensures that target features stay in the scanned regions, thereby drastically improving signal quality. However, advancing technologies will in the very near future allow us to employ fast real-time motion correction solutions as well. These will be able to eliminate motion artifacts during data acquisition not just along the axes of the imaged plane, but also along the optical axis. Using this technology, researchers will be able to acquire neuronal activity data while the animals are moving in virtual reality and performing various tasks, profit from unprecedented SNR, and save time by avoiding time-consuming post-hoc processing.
The Great Potential of Voltage Imaging
Two-photon microscopy is still a comparatively young branch of technology in the field of neuroscience, and along this branch, new and promising biotools are budding continuously. 20 years ago, scientists used chemical dyes (mostly OGB-1) on a daily basis to visualise the activity patterns of individual neurons. Back then, this was an effective tool, injected into single cells or slice preparations (called bulk loading) (9) – however, researchers using it faced obvious difficulties: for instance, you could only perform acute imaging, patchclamping, or the imaging of a limited number of neurons. The limitations were even more pronounced during in vivo behavioural experiments. This changed rapidly after the Ca2+-sensing molecule, G-CaMP, was first reported in The Journal of Biological Chemistry (9). This molecule allows for the imaging of thousands of neurons, even in the deepest brain regions during complex behavioural experiments. Approximately ten years ago, almost every lab started to use GCaMP as their main dye because of these significant advantages. However, GCaMP has some disadvantages as well, such as slow kinetics, photobleaching sensitivity, and its inability to give direct membrane potential changes, due to being a second messenger. GCaMP cannot resolve single action potential (AP) or sub-threshold signals which would be crucial for sophisticated dendritic measurements.
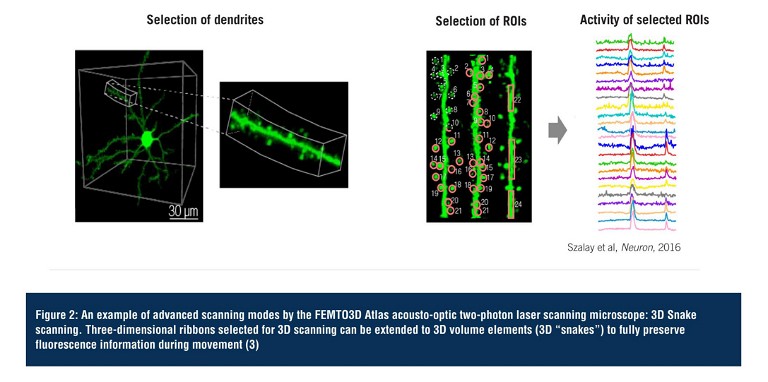
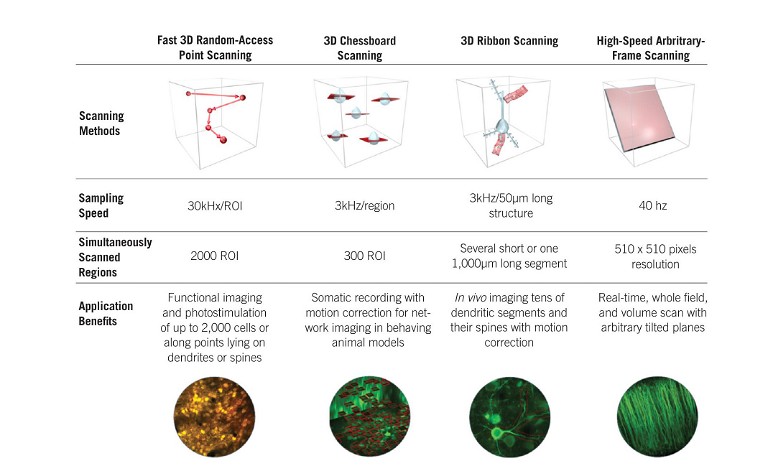
Figure 3: Features and advantages of the advanced scanning modes of the FEMTO3D Atlas acousto-optic two-photon laser scanning microscope
The FEMTO3D Atlas has two extremely important advantages if paired with a suitable working voltage sensor. The first one is the high sampling rate range (up to 30kHz) which makes it suited for detecting ultrafast transients, like APs. Currently, one of the most established scanning technologies is using resonant-galvo scanners for in vivo imaging. However, they are simply too slow in the raster scan mode to resolve these electrical signals. The AO technology can overcome this problem, as it surmounts mechanical distortion and jumping delay between ROIs: it only records valid fluorescence signals. The second advantage of an AO system in this context is the previously mentioned real-time motion correction solution, which could, and most likely will, be a real gamechanger in direct voltage imaging. These promising possibilities might soon usher in the beginning of a new era in neuroscience.
References
1. Grienberger C and Konnerth A, Imaging Calcium in Neurons,
Neuron, 8;73(5): pp862–85, 2012. 2. Peterka DS, Takahashi H, and Yuste R, Imaging voltage in neurons, Neuron, 69(1): p9, 2011. 3. Szalay G et al, Fast 3D Imaging of Spine, Dendritic, and Neuronal Assemblies in Behaving Animals, Neuron, 92(4):723–38, 2016.
4. Helmchen F and Denk W, Deep tissue two-photon microscopy, Nature Methods, 2(12): pp932–40, 2005.
5. Katona G et al, Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes, Nat Methods, 9(2): pp201–8, 2012.
6. Geiller T et al, Large-Scale 3D Two-Photon Imaging of Molecularly Identified CA1 Interneuron Dynamics in Behaving Mice, Neuron, 108(5): pp968-983.e9, 2020.
7. Geiller T et al, Local circuit amplification of spatial selectivity in the hippocampus, Nature, 601(7891): pp105–9, 2022. 8. Visit: femtonics.eu/femto3d-atlas 9. Ji G et al, Ca2+-sensing Transgenic Mice: Postsynaptic Signaling In Smooth Muscle, Journal of Biological Chemistry, 279(20): pp21,461–8, 2004.

Dénes Pálfi, Head of the Application Specialist team at Femtonics, has a PhD in neuroscience and has been working with conventional and high-end multiphoton microscope imaging technologies for more than ten years. Although his main interest is physics, he has spent a considerable amount of time gathering knowledge in the field of neurobiology and programming. After his PhD, he started to focus on industrial processes instead of research and continued his career at Femtonics as an Application Specialist.