Product News – Winter Edition
IPT highlights some of the most recent exciting advancements in pharmaceutical manufacturing
CARBOGEN AMCIS announces successful refinancing and enhancement of their syndicated credit facilities to support continued growth and investment
IPT highlights some of the most recent exciting advancements in pharmaceutical manufacturing

CARBOGEN AMCIS, a global top-tier contract development and manufacturing organisation, announced the successful refinancing and enhancement of its credit facilities through the banking syndication led by UBS Switzerland AG. The transaction, led by UBS Switzerland AG with the participation of several leading financial institutions in Switzerland, reflects the strength of CARBOGEN AMCIS’s long-term relationships with its banking partners and their confidence in the Group’s business strategy and future growth trajectory. “The successful refinancing marks another important step in CARBOGEN AMCIS’s growth journey. It demonstrates the confidence our banking partners have in our long-term strategy and strengthens our ability to invest in the technologies and infrastructure that will support our customers’ most advanced development programmes. While our initial investment round supported the expansion of our Drug Product operations in France, we are now focused on broadening our technological capabilities across the global organisation, positioning CARBOGEN AMCIS at the forefront of innovation and delivering greater value to the pharmaceutical industry,” said Stephan Fritschi, CEO of CARBOGEN AMCIS.
Primerdesign launches exsig Mag RapidBead Pro Extraction kit for DNA and RNA

Primerdesign, a company focused on the design, manufacture, validation and supply of real-time PCR kits and reagents, today announced the launch of its exsig Mag RapidBead Pro Extraction kit. The next-generation magnetic bead-based kit can be used to extract high-quality DNA and RNA from diverse sample types. The streamlined and adaptable protocol enables efficient nucleic acid purification, helping researchers to move faster from sample to PCR testing, without compromising on yield and purity.
Abselion launches his-tagged Protein Quantification kit and sensors for Amperia at PEGS Europe 2025

Abselion, a pioneering life sciences technology company focused on simplifying biomolecule quantification, has expanded its Amperia assay portfolio with the launch of its Tagged Protein Quantification Kit, His-tag and Tagged Protein Sensor, His-tag Quantification. The novel assay, developed with GenScript’s THE His Tag monoclonal antibodies, provides researchers with a ready-to-use solution for rapid, reliable measurement of His-tagged proteins. This launch marks a significant milestone in extending Amperia, beyond antibody and adeno-associated virus assays, into the wider field of recombinant protein analysis, enabling researchers to generate high-quality data more quickly and dependably across expression, optimisation and development workflows. Each kit includes pre-coated sensor strips, together with assay plates, detection reagents, and buffers in a complete, ready-to-use package. At the core of the new kit are electrochemical sensor strips pre-coated with GenScript’s validated antibodies, immobilised on the sensor surface. These high affinity antibodies offer broad reactivity across common His-tag variants and expression systems for enhanced assay robustness. Incorporating these directly onto the strip reduces manual handling steps and the need for additional assay development or optimisation, improving usability and supporting more consistent performance.
Natoli Engineering offers Precision CT Scan for non-destructive insights into internal tablet structuresa
Natoli Engineering, a globally recognised innovator in the tablet compression industry, features Precision CT Scan. This 2D and 3D imaging solution provides non-destructive insight into internal tablet structures, helping detect flaws and avoid costly problems. Early detection helps maintain quality, ensure compliance and protect manufacturers’ reputations.
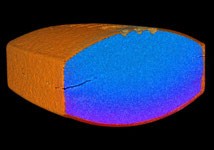
Natoli Engineering’s Precision CT Scan delivers non-destructive 2D and 3D imaging that reveals internal tablet defects such as voids, cracks, lamination, density inconsistencies and improper layering. It measures coating thickness, maps multi-API distribution, and detects foreign materials like metal or plastic. Precision CT Scan can inspect blister packs and sealed vials without damaging seals to verify tablet integrity, count and alignment. It easily integrates into a wide range of manufacturing environments and supports quality assurance and regulatory professionals. The user-friendly, scientifically accurate Precision CT Scan improves blend uniformity, coating accuracy and press calibration, while validating metal detection. This reduces recalls and batch rejections and helps manufacturers meet regulatory standards. Our experts can provide a customised walkthrough.
GE HealthCare’s Photonova Spectra with Deep Silicon reimagines photon-counting CT to see more, know more and achieve more
GE HealthCare announced the submission of a 510(k) to the US Food and Drug Administration seeking clearance for Photonova Spectra, its new photon-counting computed tomography system with advanced artificial intelligence algorithm. Built on GE HealthCare’s proprietary Deep Silicon detector technology, Photonova Spectra is designed to deliver remarkable spectral and spatial resolution for ultra-high-definition (UHD) imaging with wide coverage, seeking to enable fast acquisition speeds, precise visualisation of anatomical structures and enhanced material separation.
Harnessing the full potential of its proprietary Deep Silicon detectors, the system’s UHD imaging is designed to deliver wide coverage, enabling fast acquisition speeds and the precise visualisation of subtle tissue variations, small lesions and vascular structures. With on-demand spectral imaging for every scan, it also seeks to empower clinicians to detect, characterise and monitor disease with confidence. Staff can also rely on one protocol setup for many exams, reducing complexity and supporting efficiency. Altogether, these capabilities aim to deliver clinicians the information they need in one exam and enable fast, confident diagnoses and treatment planning.
ARPA-H launches programme to fund autonomous stroke-treating robots

The US government’s Advanced Research Projects Agency for Health (ARPA-H) has launched a new funding programme aimed at the development of autonomous robotics – including a special focus on systems designed to intervene in cases of stroke, as well as a broader remit covering a range of surgical procedures that could potentially be performed without human hands. The agency, known as ARPA-H, is seeking proposals from organisations with expertise in surgery, imaging and artificial intelligence, including academic and scientific research institutions as well as the private sector, and is encouraging applicants to form multi-disciplinary teams. ARPA-H, which first launched in late 2022, places stroke as one of the top causes of death and disability in the US. The agency further estimates that most people in the country live too far away from properly equipped and staffed centres to undergo the life-saving procedures necessary to remove blood clots from the brain. At the same time, for less urgent procedures such as biopsies and kidney stone removals, ARPA-H said the future development of microrobotic systems could deliver less invasive care without specialised equipment.