SPOT LIGHT
X-ray CT for tablets: a powerful non-destructive tool for tablet characterisation
How is X-ray CT being used to ensure quality in tabletting?
Rahul V Haware, Robert Sedlock, Grant Sedlock at Natoli Scientific; Ming Ji at the University of New Mexico College of Population Health; Nitin kumar Swarnkar and Sandip Tiwari at the BASF Corporation; and Angela Criswell at Rigaku Corporation Americas
The tablet is the most popular vehicle for well-controlled, targeted and predictable drug release.1 It offers a consistent and large volume production at relatively low cost with minimum waste. Tablets are composed of active pharmaceutical ingredients (API) and inactive ingredients or excipients. Thus, tabletting properties can be an API driven (API >50%), an excipient driven (excipient >90%) or both API-excipient driven (API >25% and excipient >75%), in addition to applied manufacturing parameters.
These parameters dictate the internal 3-dimensional (3D) tablet skeleton. The changes in microscopic and macroscopic morphology of tablet ingredients and their distribution after compression process across the 3D tablet skeleton dictates the intrinsic and extrinsic tablet attributes and performance. The intrinsic attributes include API and excipients content distribution, pore concentrations, granule morphology, and presence of impurities and solvents. The extrinsic attributes include mechanical strength, coating layer thickness and stability. Extrinsic tablet properties are direct implications of intrinsic properties and resulting tabletting performance.
Traditional testing methods
Various official pharmacopoeial tests are employed to ensure consistent qualities of tablets. However, a traditional surrogative off-line tablet testing could not guarantee consistent tablet qualities as well as detailed insights on a tablet’s internal 3D skeleton required to decode performance failure modes. Poor tablet characterisation could result in poor tablet functionality, which could result in the failing of drug approval or assessment tests, and possible tablet recalls of approved product from the market.2 Therefore, assessing intrinsic and extrinsic characteristics and ensuring their uniformity within the production series is particularly important for the drug development, quality control of optimised manufacturing process, and approval from regulatory agencies like the US Food and Drug Administration (FDA) and European Medicines Agency.
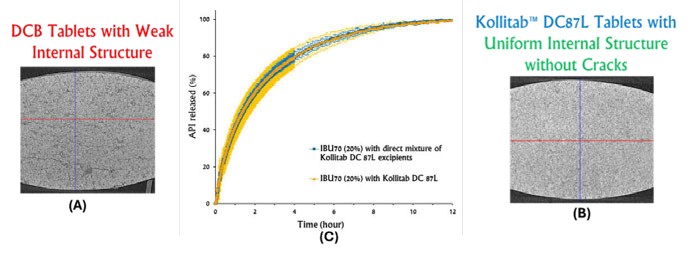
Figure 1: Impact of internal tablet structure of (A) tablets of physical blends of Kollitab DC 87L and (B) Co-processed Kollitab DC87L on (C) ibuprofen release profiles
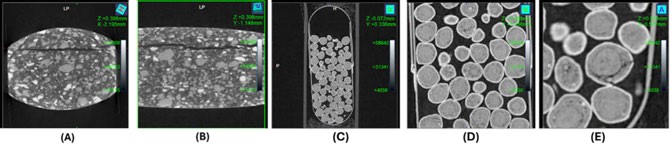
Figure 2: Tablet capping (A) Side and (B) Front view and X-ray micro-CT scans of (C) OTC Lansoprazole capsule, (D) Lansoprazole granules and (E) Lansoprazole granule defects
The FDA ICHQ8 pharmaceutical development and ICHQ9 quality risk management guidelines have provided various advanced spectroscopic techniques to assess, monitor and manufacture in-built quality tablets.3,4 These spectral techniques include near-infrared, infrared and Raman spectroscopy. However, though these non-destructive techniques provide in-depth chemical profiling of tablets, they do not provide the tablet’s internal 3D physical skeletal information.
Introducing X-Ray CT
A high-resolution X-ray computerised tomography (X-ray CT) provides a non-destructive detailed analysis of density gradients and internal defects such as cracks and voids, as well as internal 3D physical structure. This is vital information during the tablet formulation and development process to decode root cause analysis of both success and failure of manufactured tablets while meeting desired specifications and performance. Furthermore, X-ray micro-CT is a simple and convenient technique for tablet characterisation, as it requires minimal to no additional sample preparation before the experimentation.
Assessing a tablet’s 3D internal physical skeleton
KollitabTM DC87L is a co-processed excipient composed of lactose monohydrate (87%), crosspovidone (9%), Kollitab® IR (3%) and sodium stearyl fumarate (1%). An X-ray micro-CT scan was used to evaluate the tabletting performance of co-processed Kollitab DC87L and the physical blend of its components. The X-ray micro-CT scan generated the 3D internal physical skeleton of this tablet and revealed that tablets made with the physical blend of Kollitab DC87L exhibited several cracks throughout the tablet body (Figure 1). Contrary, the co-processed Kollitab DC87L did not show any cracks. These findings exhibited the better tabletting performance of co-processed Kollitab DC87L as compared to the physical blend of its components. This better tabletting performance of Kollitab DC87L could be attributed to better material deformation of co-processed excipients during the compression cycle.
Detecting tablet defects
A commercial manufacturing of tablets poses four major tabletting defects – lamination, capping, picking and sticking. Capping involves separation of the top or bottom part of the tablet from the main tablet body during the ejection cycle. Tabletting material picking occurs at engraved areas like logos or letters of the punch surface, which causes impression defects on the next tablet. Lamination is another important tabletting defect involving the splitting of tablets into two or more layers parallel to its flat faces. Sticking is a major tabletting problem that involves tabletting materials sticking to the punch surface due to more excessive adhesive forces occurring between the punch face and material than cohesive forces between the tabletting materials. These problems are prominent in the late development stage due to large production batch size. Such problems require a solution without changing the formulation due to regulatory constraints.
Natoli Scientific has generated in-house analytical methodology for both tablet sticking and capping.5 These material-sparing methodologies are based on powder rheological and deformation properties. The proposed methodologies allow the finding out of root causes of tablet sticking and capping, and provide possible pragmatic solutions without changing the formulation.
Coupling of X-ray micro-CT scan with the Natoli in-house capping and sticking methodologies could serve as a powerful tool for root cause analysis of such tabletting problems. Importantly, X-ray micro-CT generated 3D internal physical structure provides clear differentiation between capping, picking and lamination. Figure 2 shows the side and front view of a tablet indicating clear separation of the top layer from the main body of the tablet. This can be identified as tablet capping. Such information is vital to optimise existing formulation by reducing the key parameters responsible for capping such as reducing in-die tablet expansion in the decompression phase or modifying tool designs.
“ A non-destructive and high-resolution X-ray micro-CT scan could provide a quantitative measurement of coating thickness, as well as the presence of pores in the coating layer ”
Correlation of tablet structure with dissolution performance
A dissolution performance of ibuprofen Kollitab DC87L and ibuprofen Kollitab DC87L component physical blend tablets were measured with USP II apparatus and 0.1N HCl (pH 1.2) as a dissolution medium. The paddle speed was 75rpm. The temperature of dissolution media was 37 ± 0.5° C. Kollitab DC87L tablets with and without 20% ibuprofen were >3 times stronger than those made with the physical blend. As mentioned previously, X-ray CT scan confirmed a strong and uniform structure of Kollitab DC87L tablets and was compared to its physical mixture tablets (Figure 1). The X-ray CT scan revealed that Kollitab DC87L tablets did not possess any internal cracks, while tablets made with the physical blend of Kollitab DC87L contained a substantial number of internal cracks. As a result, the ibuprofen release from Kollitab DC87L tablets had less variability than that from tablets made with the physical blend of Kollitab DC87L (Figure 1C).
Granule size estimation in capsules
The multi-unit pellet system (MUPS) is a multi-particulate system designed for immediate release, modified drug release and to administer incompatible drugs. These dosage forms contain uncoated as well as coated pellets of functional coatings with variable thickness to achieve desired modified drug release. Therefore, core granule or pellet size distribution and their porosity can affect the modified drug release profile; a pellet with smaller size distribution and high porosity could lead to a rapid disintegration and faster drug release than desired criteria. X-ray micro-CT scans could be a powerful technology to evaluate these features of MUPS. Figures 2C and D show OTC lansoprazole MUPS capsules. These scans reveal varying granule size distribution, viscosity, as well as broken granules (Figure 2E).
Coating uniformity of functional coatings
Tablets are coated for various purposes like protection, taste masking or controlled release. One of the critical parameters of coating layers is coating thickness, uniformity and its distribution. A typical tablet coating thickness varies between 5-100µm. The coating process impacts the coating layer uniformity. Typical industrial coating process uniformity is monitored with tablet weight gain, scanning electron microscopy and with dissolution testing. However, these non-destructive and destructive tests and indirect parameters do not provide real measurements of coating thickness. A non-destructive and high-resolution X-ray micro-CT scan could provide a quantitative measurement of coating thickness, as well as the presence of pores in the coating layer.
Coating uniformity of multi-unit multi-particulate system
Coated pellets along with non-coated pellets or granules are key components of multi-unit multi-particulate systems. These coated pellets or granules have appropriate coating film made with the desired type and amount of polymer. A variation in coating thickness, as well as coating integrity during the shelf life, is important to achieve modified drug release. A non-destructive and high-resolution X-ray micro-CT scan could provide a qualitative and quantitative measurement of coating features. It can provide coating integrity as well as coating layer thickness measurements.
Conclusions
The present study has demonstrated application of a high-resolution X-ray micro-CT as a powerful tool for various solid dosage form characterisations. This non-destructive technology can provide direct correlation of a tablet’s internal structure on the final dosage form performance. It can also serve as a direct methodology for a quantitative evaluation of various functional and non-functional coating features as compared to currently applied indirect methods. Recently, Natoli Scientific has acquired this powerful technology to provide our national and international clients the visibility of invisible features of manufactured solid dosage forms.
References:
- Lieberman, H.A et al (1980), ‘Pharmaceutical Dosage Forms: Tablets’, Marcel Dekker Inc, Volume 1
- Bostijn N et al (2018), ‘In-line UV spectroscopy for the quantification of low-dose active ingredients during the manufacturing of pharmaceutical semi-solid and liquid formulations’, Analytica Chimica Acta, 1013, 54-62
- Visit: database.ich.org/sites/default/files/Q8_R2_ Guideline.pdf
- Visit: fda.gov/regulatory-information/search-fdaguidance-documents/q9r1-quality-risk-management
- D Patel KC et al (2021), ‘Powder Rheology: Gateway for Tablet Sticking Insights’, Manufacturing Chemist

Dr Rahul Haware is director of Scientific Support and New Business Development at Natoli Scientific, leading the Preformulation team. He applies pharmaceutical material science, process analytical technology, design of experiments and quality-by-design principles to develop robust formulations in early drug development, reducing late-stage manufacturing issues. Dr Haware holds Master’s degrees in Pharmacy and Molecular Biology and a PhD in Powder Compaction Physics from UiT The Arctic University of Norway, Norway. He was a professor for eight years, and has authored three book chapters and over 46 peer-reviewed papers.

Grant Sedlock is a technical representative at Natoli Scientific with a background in solid dosage development, manufacturing, quality control, and strain gage instrumentation systems. Grant’s area of expertise also includes troubleshooting common tablet compression issues through micromeritic analysis, USP<1062> tablet characterisation profiling and x-ray computed tomography. Grant is an invited instructor at the University of Mississippi (Hands-On course on Tablet Technology) and the University of Maryland (Tablets & Capsules Hands-On short course), both US.

Robert Sedlock is the director of Natoli Scientific and brings over 25 years of industry experience. His early career focused on strain-gage technology and data-acquisition systems for government stress-testing, later adapted for pharmaceutical manufacturing equipment. Since joining Natoli Engineering in 2015, he has collaborated with graduate programs to advance solid-dosage training and industry research. In 2018, he opened Natoli Scientific’s 14,000sqft Telford, PA, US facility, which provides training, contract R&D and formulation services. Robert is a frequent invited speaker worldwide and has authored numerous technical articles and peer-reviewed papers.

Dr Ming Ji PhD is a professor of Biostatistics at the University of New Mexico College of Population Health, US. He earned his PhD in Biostatistics from UC Davis, US, an MS in Mathematics from Kansas State University, US and a Master’s in Applied Mathematics from East China Normal University, Shanghai, China. Dr Ji specialises in advanced statistical methods for longitudinal data, clinical trials, complex surveys, causal inference and missing-data problems. His research focuses on health behaviour, cancer prevention, microbiome studies and public health interventions. He has authored over 100 peer-reviewed publications, collaborated on National Institute of Health and Center for Disease Control-funded studies, and trained researchers in biostatistics and clinical trial methodology.

Dr Nitin Kumar Swarnakar is a seasoned professional with over 16 years of experience in oral and parenteral drug product development. He currently holds the position of North America Application lab manager at BASF Corporation, US, and heads the pharma solution lab. Dr Swarnakar earned his PhD in Pharmaceutical Science from the National Institute of Pharmaceutical Research and Education in Mohali, India, and holds BPharm and MPharm degrees in Pharmaceutical Sciences from Dr HS Gour University, Sagar, India.

Dr Sandip B Tiwari is head of Technical Services – Pharma Solutions North America at BASF Corporation, supporting biopharmaceutical companies. He previously served as fellow in Manufacturing Science & Technology at Teva Pharmaceuticals and as senior manager and Technical Director at Colorcon, focusing on innovative modified-release systems. He also worked on novel drug delivery systems at Zydus Cadila and completed a postdoctoral fellowship at Northeastern University, UK, on nanotechnology-based biopharmaceutical delivery. Dr Tiwari earned his PhD in Pharmaceutical Sciences from Manipal, India, and has authored a book, nine book chapters, and over 100 publications, patents and abstracts in dosage form design, modelling, AI/ML and excipients.

Dr Angela Criswell PhD is director of X Ray Imaging at Rigaku Corporation Americas, where she leads advanced imaging and materials analysis efforts. She earned her PhD from Rice University, US, and joined Rigaku in 2002. Over her tenure she has developed deep expertise in techniques including small-angle X ray scattering and X ray CT and uses these tools to support research and quality control across industries from biotechnology to materials science. Dr Criswell is a recognised thought leader who regularly shares insights through webinars, articles and technical trainings to help customers tailor imaging solutions to their scientific challenges.