Manufacturing: Gene Therapies
Innovative manufacturing techniques for gene therapies
What are some innovative techniques used for manufacturing gene therapies at a commercial scale?
Ian Aled Jones at FinVector Oy
Large-scale vector manufacturing for commercial production has proven to be challenging, especially if the treatment regime is to provide the patient with a high dose virus load.1,2 These requirements have led to innovative manufacturing techniques for novel manufacturing solutions of gene therapy at a commercial scale.
It is clear that gene therapy is the future of medicine and will be the only therapeutic solution to many unmet patient needs, however the challenge now lies with taking these potential therapies that can be produced at very small scale in academic institutions and scaling them up to commercial cost-effective medicine. Currently, only a very small number of organisations have the sufficient high capacity manufacturing capability to manufacture viral vectors at a cost-effective commercial scale. This has been undertaken through investment in high-capacity manufacturing with specially trained and skilled associates under highly specific conditions, using precision equipment and advanced facilities. More critical is that the organisation fully understands and supports the emerging regulatory standards around the world in order to fulfil global supply chain needs for gene therapy medicinal products, a subsection within the advanced therapy medicinal products classification.
The critical elements for a successful commercial-grade process include the development of a platform approach that is easily scalable from small laboratory systems through to a full commercial-scale operation, and the existence of a strong partner network between the manufacturing organisation and the supplier of the equipment and single use components. Many gene therapy products have failed to demonstrate required bioequivalence at late-stage submission when preclinical studies and toxicology were undertaken using a completely different manufacturing methodology.
Analytical methods
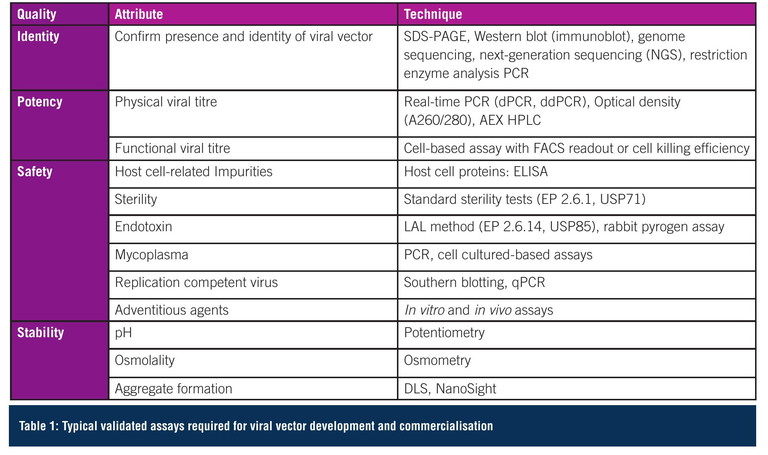
Not only does vector manufacturing require to be commercially realistic, it is critical that the analytical methods used in developing the manufacturing process are validated, accurate and reproducible so quality attributes can be monitored – ensuring a safe, high quality, consistent and efficacious product. There are very few monographs that establish limits for quality and purity, so defining release specifications for viral vector products can be challenging and experience in this field is imperative. A typical set of assays for identity, potency, safety and stability is shown in Table 1. Furthermore, standardised reference materials are rarely commercially available, therefore the organisation must develop and validate a set of reference standards prior to the clinical phase, or potentially use assay controls to support the demonstration of assay consistency.
Process typical viral vector manufacturing
There is a large range of producer cells that can be used for viral vector manufacturing, with human embryonic kidney 293 (HEK293) or PER.C6 typically being favoured as they produce high virus titre. Through combining all the necessary components for viral protein expression, assembly and packaging of the therapeutic gene in the producer cell line, efficient vector production is ensured. Although producer cells have been grown efficiently in both adherent and suspension mode, not all cells can be efficiently grown in large-scale suspension bioreactors. This has led to a preference for adherent production systems for efficiency of scale-up as the ability to perfuse the media across the anchored cells allows similar cell growth conditions between small and commercial systems.
Most academic research is based on flask-type 2D approaches and scaled up by introducing more flasks. However, trying to scale up production using this methodology is very limited due to the incubator space requirement in production areas. Another issue is the need to monitor growth metabolites in multiple units, due to the need for manual manipulation making it very labour-intensive. Also, from a regulatory perspective, it is very difficult to ensure uniformity and control across the production batch in multiple flask units. Most commercial viral vector platforms are typically either single use adherent fixed-bed or suspension mode bioreactors, with small-scale models allowing scale up from laboratory to full scale. Once the vector is manufactured at commercial scale, the most significant technical challenge is ensuring the material is not lost in the downstream process, therefore at a commercial scale a high yield of around 80% would be desired.1 The transition from laboratory to commercial production typically requires replacement of low-volume ultracentrifuges with tangential flow filtration (TFF), chromatography and larger bioburden reduction filtration systems where process understanding is critical, and again any change from the initial process design will have a regulatory impact. To be able to achieve high yields, the downstream purification process should have as few unit operations as possible with a very sophisticated downstream process buffer system.
There are significant advantages to working with a buffer system that enables processing in high concentrations, as it reduces the working volumes and processing times across the process. However, an understanding of the techniques is critical as product aggregation issues are common when working with high buffer concentrations. Finally, development of a suitable final formulation buffer is essential for maintaining product stability, functionality, and quality during storage conditions and times.
The viral vector-manufacturing process starts by thawing the producer cell bank vial (Figure 1). Cells are expanded to the inoculation target cell number in suspension mode with flasks, then roller bottles and finally in a wave suspension bioreactor using defined media. The cells are then inoculated into the bioreactor for adherent cultivation in with media perfusion. Typically, cells are cultivated at a temperature of +37°C, with a range of dissolved oxygen 50% (air saturation) and at a neutral pH.
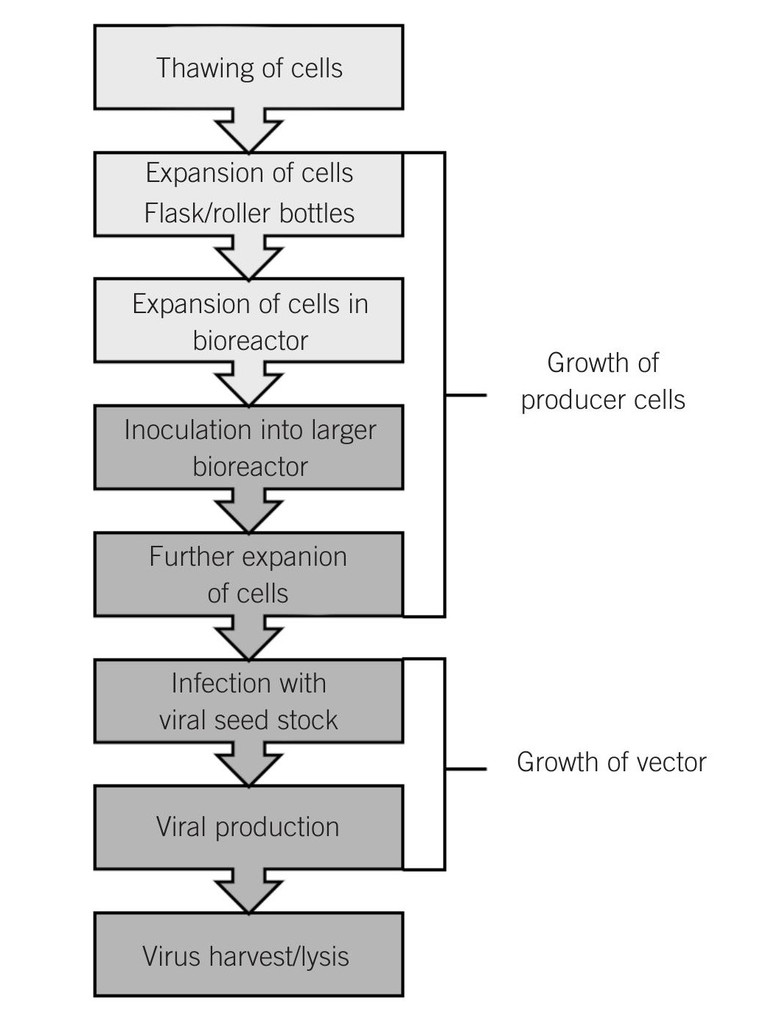
Figure 1: Upstream viral vector manufacturing process
When cells are at the target cell density as defined by a fixed cell-doubling time, viral vector production is induced by adenovirus infection, utilising a defined multiplicity of infection (MOI). Virus generation typically takes just over two days, and then the product is harvested by lysing the cells to release the virus and collecting the lysed harvest. The chosen control strategy is to add a fixed number of cells at inoculation and have a fixed cultivation growth duration with a constant doubling time to determine the total cell number at moment of infection. Infection is applied with sufficient excess of infectious viral particles per cell to ensure that essentially all cells are rapidly infected upon introduction of the virus. Harvesting is initiated before viruses would naturally lyse the cells, to ensure controlled process and high product quality by preventing possibility of secondary infections in the bioreactor.
The typical downstream process consists of clarification, concentration, purification, polishing, and final formulation steps utilising depth filters, TFF and chromatographic techniques (Figure 2). One of the main difficulties with downstream processing is the adequate removal of aggregates and cell debris through a bioburden reduction filter to ensure that monoseptic conditions are met. To reduce fouling a balance needs to occur with the filter size and set up and system cost. The use of an endonuclease additive as well as removing residual nucleic acids typically reduces the filter-clogging propensity of the downstream material by breaking up the mucoid residues, however the removal of this enzyme material as a drug residue must then be demonstrated and proven prior to product release.
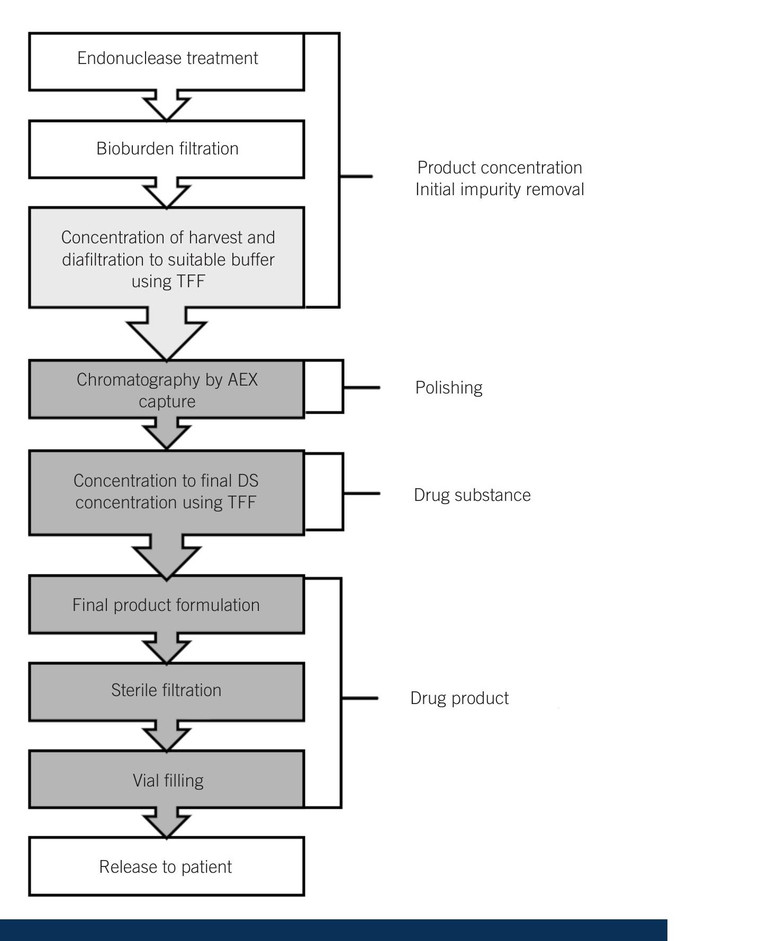
Figure 2: Downstream viral vector manufacturing process
TFF has been widely used in bioprocessing, as it can be used for both the removal of initial debris and later for diafiltration and buffer exchange to remove process-originated chemical impurities. This process is very scalable, with critical process parameters such as permeate flux rates, cycle time, monitoring of feed, retentate and permeate being tightly controlled. Further polishing of the material typically involves anion exchange (AEX) chromatography to bind product-related and process impurities. This is a scalable purification platform with many chromatography resins, which are packed into chromatography columns being commercially available. AEX separates by surface charge, with a positively charged resin selectively immobilising negatively viral particles. These are eluted along a gradient from low salt to high salt, however there is a fine balance as viral particles are impacted by high salt conditions requiring subsequent buffer exchange through diafiltration. It is critical that the process development team has a full understanding of all the process variables to optimise the product purity for the highest product yield for the downstream – this practical knowledge is something that only comes with many years of experience and is enhanced by a constant review of all the production data from continuous process validation (CPV). The review of data through advanced analytics supports optimising processes to maximise purity and yield, reduces variability and gives very predictable outcomes for each commercial batch.
Regulatory expectations
It is recognised that the manufacturing techniques and facilities required for gene therapy are far more complex than those for small molecule, and standards and guidelines for manufacturing gene therapies are constantly evolving, which complicate scale-up and regulatory approval. The primary methodology for gene therapy manufacturing is to use closed systems to minimise any risk of contamination from mycoplasma, mycobacteria or retrovirus, which allows most operations to be undertaken in Grade C cleanrooms for cost and efficiency.
“ For successful innovative manufacturing techniques for gene therapies, it is recommended that the organisation has a strong understanding of regulatory expectations, and that the site is fully current good manufacturing practice-compliant from supplier management, raw materials, storage, warehousing and incoming quality control, through to validated analytical methods and validated equipment ”
Single-use technology allows all the manufacturing systems to be sterilised by irradiation, again allowing a more flexible and scalable process – reducing downtime between product change and eliminating risk of product cross-contamination, as there are no reusable product-contact parts. For example, current regulatory expectation is for the vector manufacturing process to be validated, with a requirement to demonstrate that the MOI, in units of viral seed particles per cell, is defined to ensure a robust product output in terms of number and quality of viral infectious units. One disadvantage that was previously identified with the fixed-bed bioreactor design was the inability to count the cells through the bed during the process, as the bioreactor is single-use.1 As fixed-bed bioreactors are essential to provide efficient production of viral vectors in adherent mode at commercial volumes for critical patient applications, alternative methods to establish cell number at point of viral infection must be developed.
Strategic partnership with key suppliers
It very important to build strategic partnerships with key suppliers of both the production equipment and single-use systems. It is critical to collaborate and leverage suppliers’ expertise to drive innovation and improve efficiency, and partner with them in developing innovative processes. The development of a strategic supplier relationship to manage shared goals and joint value creation will ensure an uninterrupted supply of components to the organisation and, ultimately, patient supply. All managed within the oversight of a stable and mature quality risk management system.
Conclusion
For successful innovative manufacturing techniques for gene therapies, it is recommended that the organisation has a strong understanding of regulatory expectations, and that the site is fully current good manufacturing practice (GMP)-compliant from supplier management, raw materials, storage, warehousing and incoming quality control, through to validated analytical methods and validated equipment. The organisation should have a focus on standardisation of viral vector manufacturing to reduce manual errors and increase efficiency. Similarly, the data from each batch manufactured should be analysed to identify potential improvements in productivity and quality attributes, enabling the organisation to work with original equipment makers to support the evolution of the technology. As the industry evolves, it is critical that the specialised skills required for viral vector manufacturing are implemented across the whole organisation, including a thorough understanding of the regulatory environment. As in life, experience is everything.
References
- Lesch H P et al (2021), ’Evaluation of the Single-Use Fixed-Bed Bioreactors in Scalable Virus Production’, Biotechnol. J, 16, 2000020
- Leinonen H M et al (2020), ’Benchmarking of Scale-XBioreactor System in Lentiviral and Adenoviral Vector Production’, Hum Gene Ther, 31(5-6), 376-384

Dr Ian Aled Jones is the director of manufacturing, science and technology, innovation, and environment, health and safety at FinVector Oy. A native of Wales, he obtained his PhD in Physics at Kings College London, UK. He is responsible for a team of over 40 people driving innovation and product, process, analytical development, control strategies and CPV for gene therapy/viral vector manufacturing. He has published papers on viral vectors, lyophilisation, vapourised hydrogen peroxide and acceptance quality limit sampling. He has presented at scientific conferences on many topics including GMP compliance. He is also an active member of the Pharmaceutical and Healthcare Sciences Society.