28 articles from this collection:

Innovations in Pharmaceutical Technology Autumn 2025

CARBOGEN AMCIS
www.carbogen-amcis.com/sterile-drug-product-cdmo

CONTENTS
The contents page for Autumn 2025!

AAPS PharmSci 360
https://www.aaps.org/pharmsci/annual-meeting

Editorial Desk Autumn 2025
As the days become shorter again, and the weather cools down after a long summer, it’s the perfect time for reflecting on the year’s subjects of interest and musing about how these might be developed in the future.

EHP AWARDS
https://ehpawards.com/

Product News – Autumn Edition
IPT highlights some of the most recent exciting advancements in pharmaceutical manufacturing
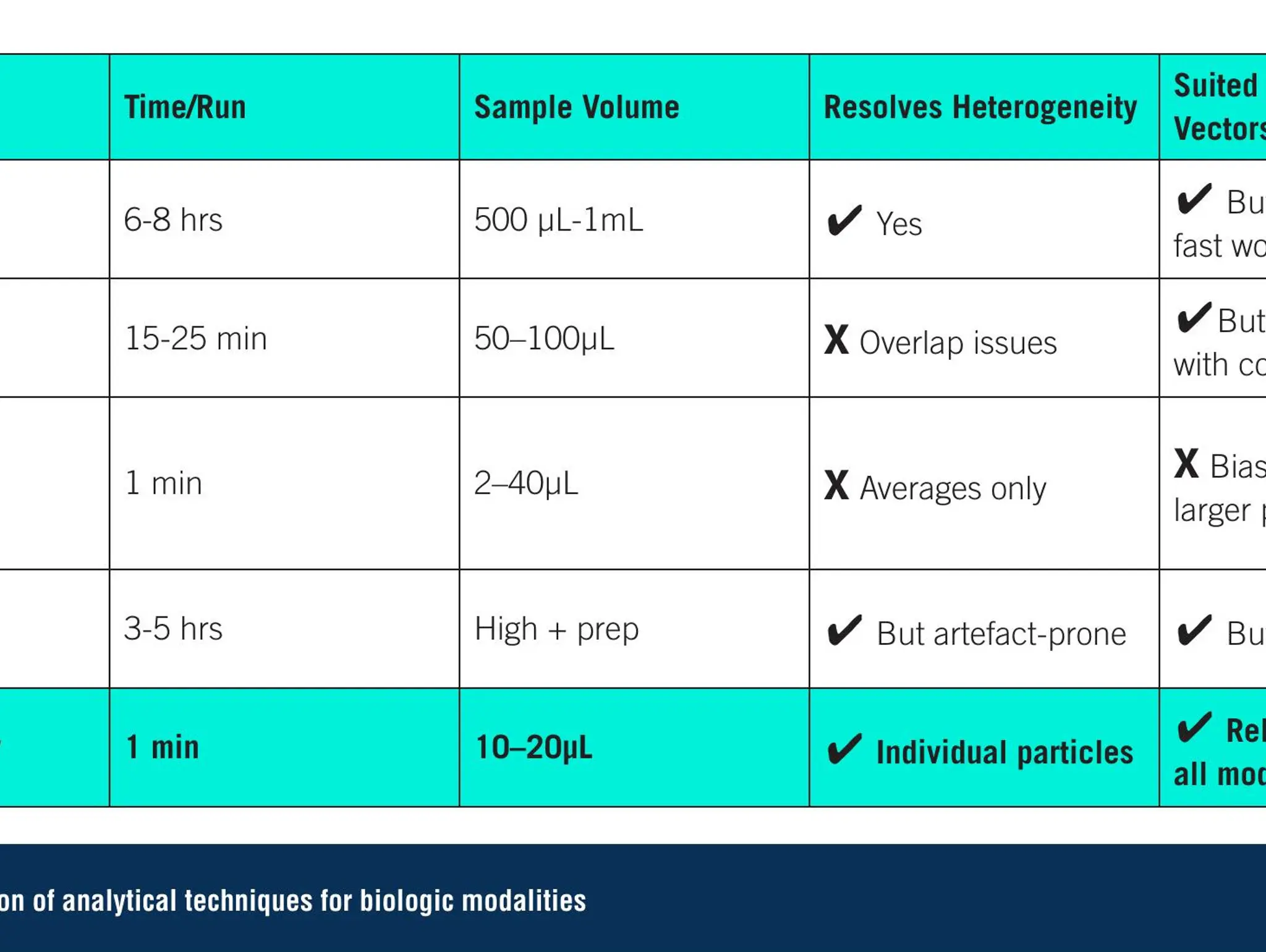
Analytics for a new therapeutic era: meeting the demands of modern biologics
As novel biologics reshape the therapeutic landscape, more advanced analytics are essential. Single-particle technologies can help fill this gap, delivering faster, clearer insights and supporting the next generation of advanced therapies
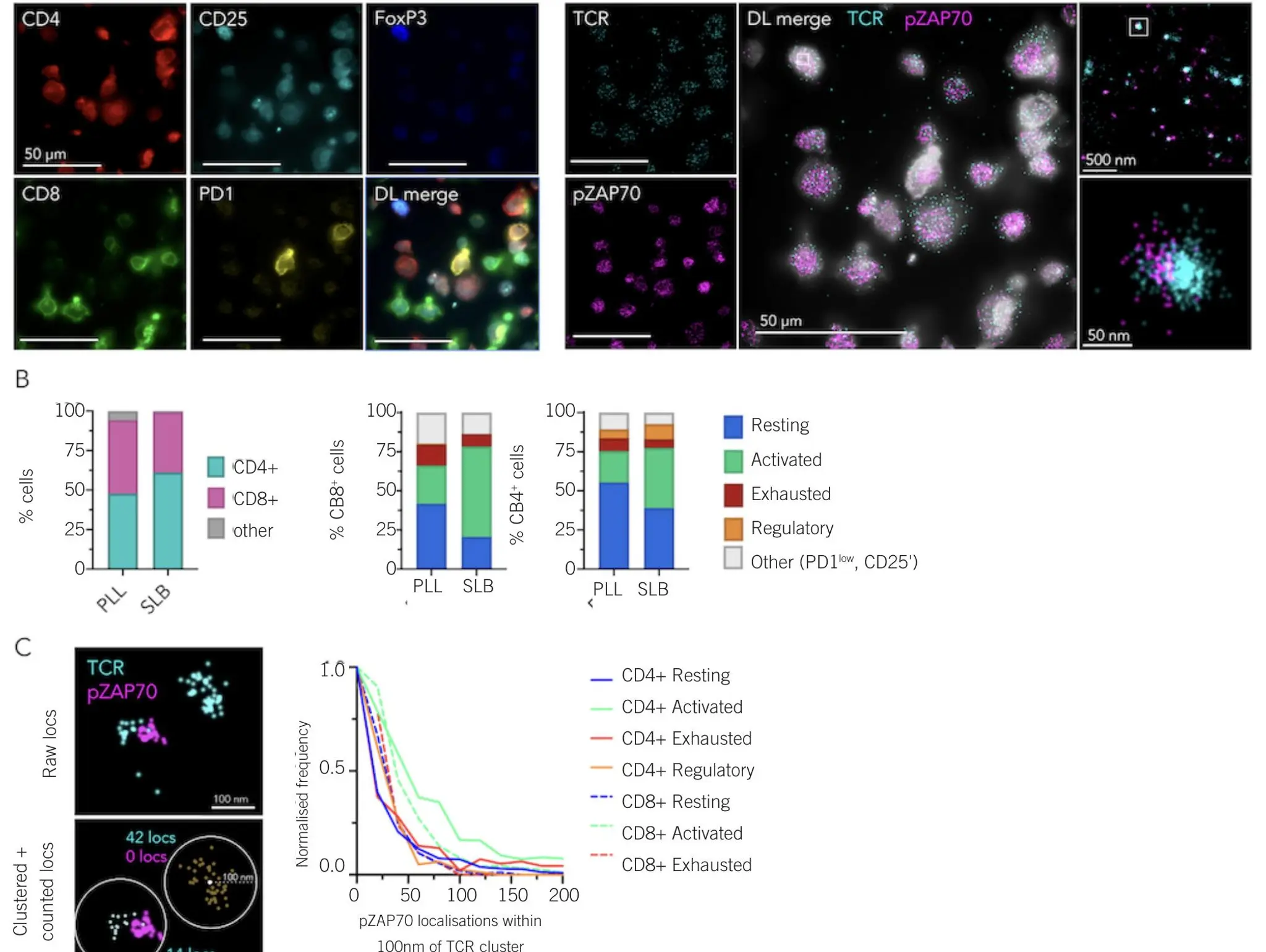
Transforming R&D workflows with automated super-resolution microscopy
What is super-resolution microscopy and how is it accelerating progress across the imaging sector?
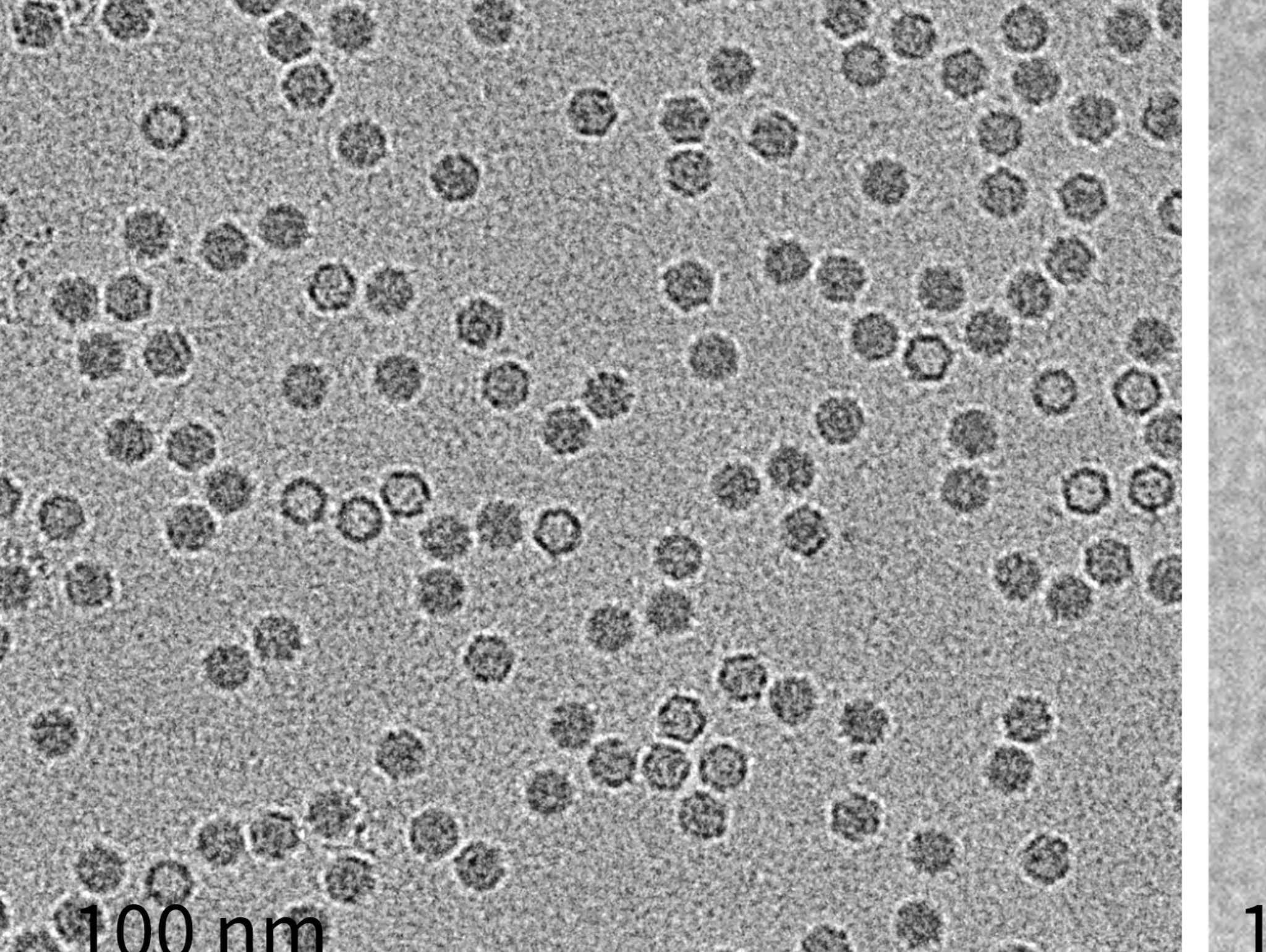
LVEM: a new tool for AAV gene therapy and pharmaceutical development
How is low voltage electron microscopy revolutionising the electron microscopy landscape – making the practice more accessible in pharma labs?
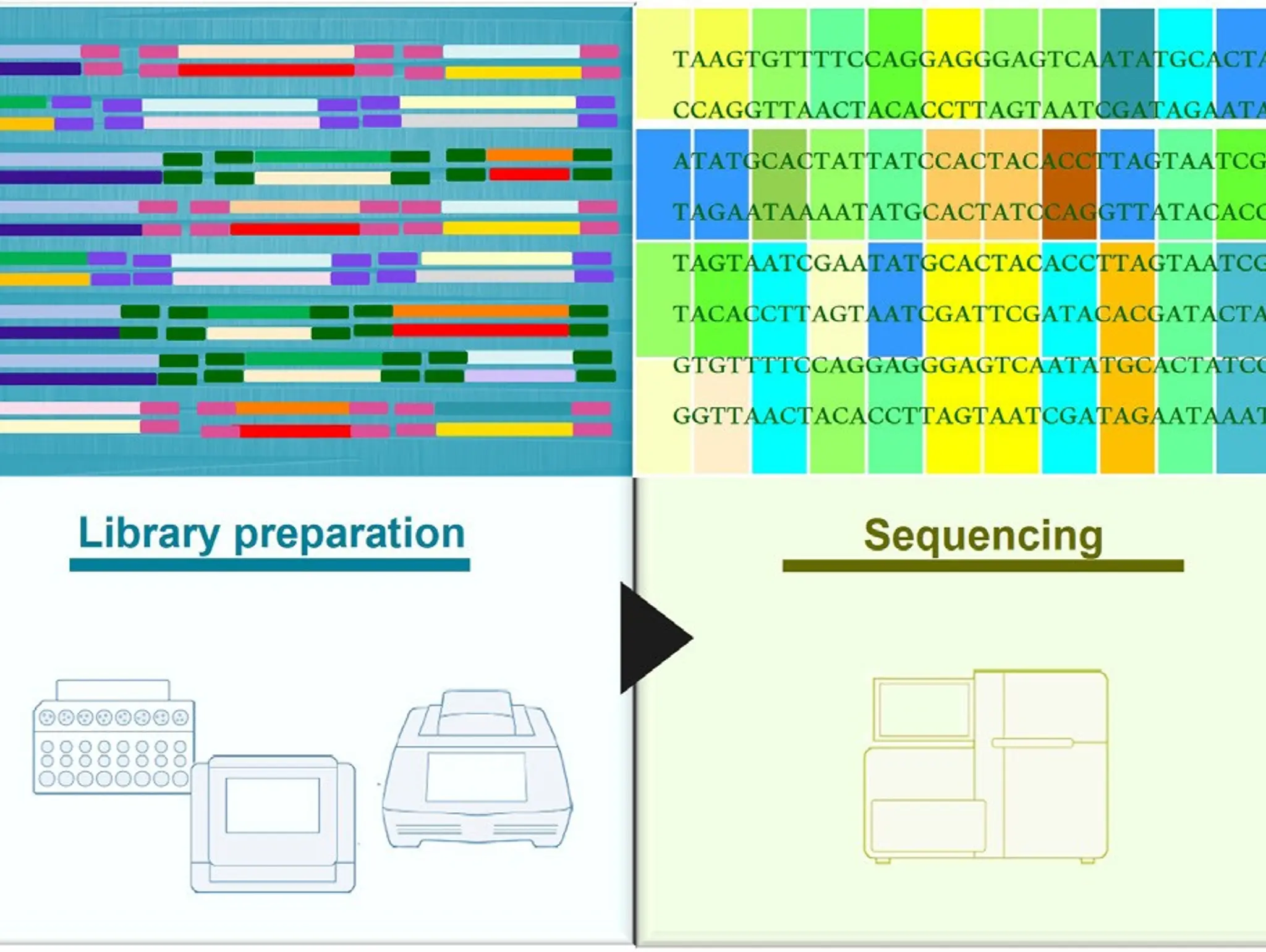
Lessons learned from sequencing: a smart beginning for meaningful results
Massive sequencing, or next-generation sequencing, has opened the door to the fascinating world of genetics, and the secret on how to use this powerful tool most effectively hinges on the starting point: the sample
Tablet density uniformity
SPOTLIGHT: Bill Supplee at Natoli Engineering discusses the best ways to ensure tablet density uniformity in pharma products
Why advanced contamination detection systems are essential for pharmaceutical manufacturing
What are eight compelling reasons why manufacturers should prioritise the use of advanced contamination detection systems in their operations?

Clone selection as a strategic lever: balancing speed, quality and long-term viability in CLD
In biologics development, clone selection is not just a technical milestone, it’s a strategic decision with far-reaching clinical and commercial implications. How can thoughtful clone selection – integrated with developability and manufacturability assessments – accelerate speed to clinic while safeguarding long-term success?

Delivering precision at every stage: integrated API and sterile drug product services
SPOTLIGHT: How CARBOGEN AMCIS supports complex molecules and challenging formulations from early development to commercialisation

Advancing complex SM process development using biocatalysis
Biocatalysis is enhancing complex molecule process development. By merging the power of enzymes with chemical synthesis, scientists and manufacturers are addressing long-standing challenges in stereoselectivity, route complexity and sustainability
The increasing pivotal role of predictive in vitro technologies in drug discovery
How will innovative human-centred in vitro testing strategies contribute to accelerating the delivery of safer and more effective therapies to patients without, or with minimal, use of animal models?
How proteomics and digital transformation can redefine therapeutic discovery
How are recent advances in proteomics, such as artificial intelligence and machine learning, transforming drug discovery and development?
Viral clearance in advanced therapies
Should viral clearance studies be required for genetically engineered viral vectors and viral vector-derived products?
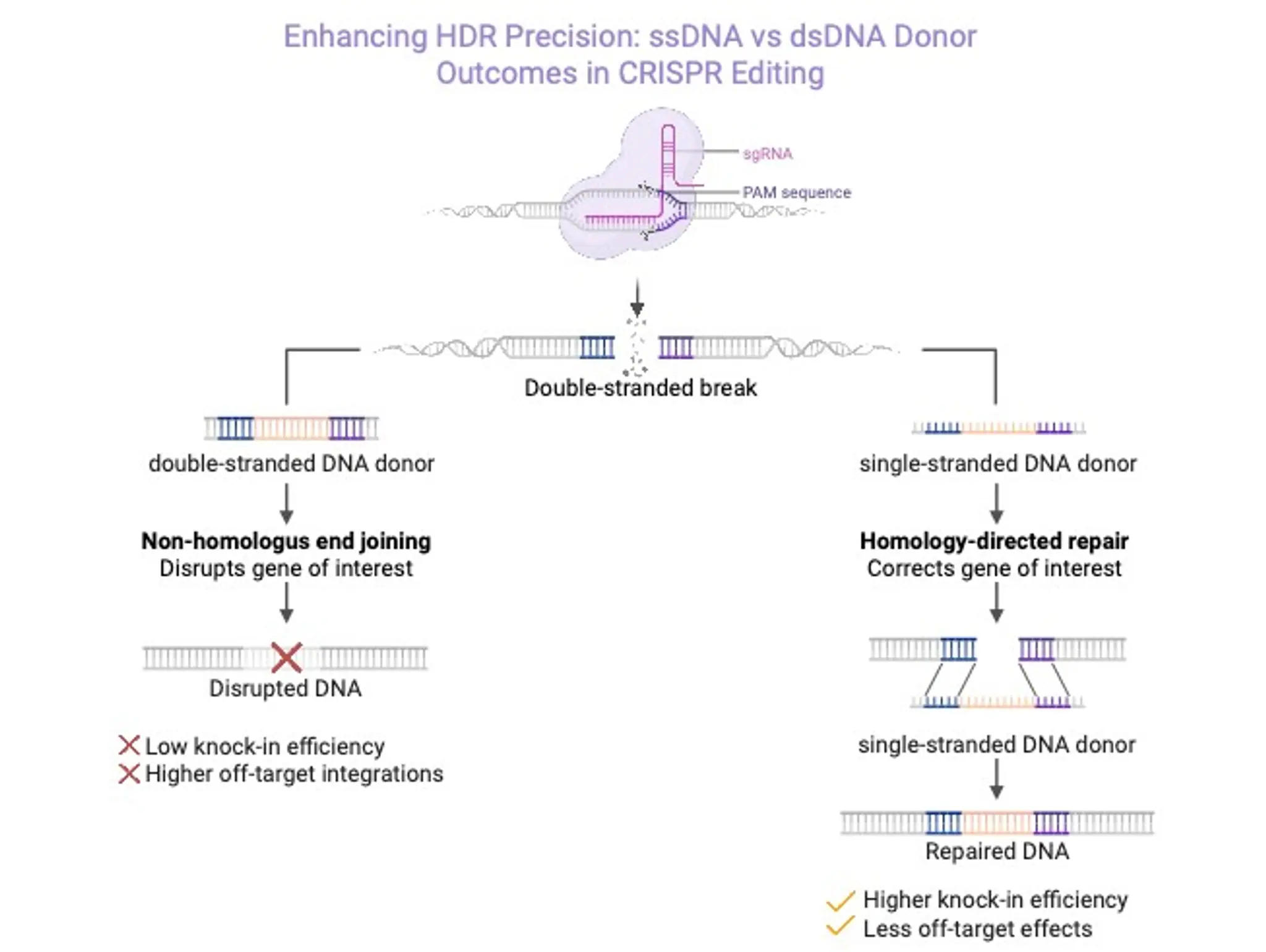
ssDNA: a novel approach to overcome efficacy and immunogenicity challenges in genetic medicines
What is single-stranded DNA and why is it a viable viral vector alternative?

Designing tomorrow’s biologics: how AI is enabling de novo protein discovery
Artificial intelligence-powered de novo protein design is opening new pathways in biologics discovery, allowing researchers to create synthetic proteins beyond nature’s existing repertoire

PMOS
https://posummit.com/

Proving value in the age of drug discovery transformation
How is artificial intelligence changing the way drug discovery and development is enacted across the sector, yielding not only impressive potential, but also clinically validated pharma products?

AI and SaaS: reshaping business outcomes in pharma manufacturing
How is artificial intelligence, when delivered through software as a service platforms, unlocking real business outcomes for pharmaceutical manufacturers, from faster batch release and smarter investigations to smoother tech transfers and enterprise-wide compliance?

Smarter labs, better discovery: how automation is evolving pharma R&D
How is the industry translating the potential of automated technologies into practical, flexible and scalable workflows that address the real-world challenges of modern laboratories, ensuring that innovation delivers measurable scientific and operational gains?

EVENTS
IPT Autumn 2025 events page

FLYPHARMA AMSTERDAM 2025
www.flypharmaeurope.com

Natoli ad Autumn 2025
natoli.com/fastlok
