Product News - Winter 2025 Edition
IPT highlights some of the most recent exciting advancements in pharmaceutical manufacturing.
16 December 2025
IPT highlights some of the most recent exciting advancements in pharmaceutical manufacturing.
16 December 2025
Nanotechnology has unlocked a new generation of hydrogels that can be tuned for specific clinical demands. From degradation kinetics to drug release and mechanical resilience, nanoscale control is transforming hydrogels from passive wound dressings into active, minimally invasive platforms for joint restoration, tissue regeneration and targeted therapeutics
16 December 2025
How is retrieval-augmented generation aiding smaller biotechs in collating and utilising disparate data systems?
16 December 2025
How is semantic layering technology addressing significant efficiency gaps, creating the infrastructure for more efficient manufacturing operations?
16 December 2025
SPOTLIGHT: Bill Supplee at Natoli Engineering discusses the best ways to ensure tablet density uniformity in pharma products
30 September 2025
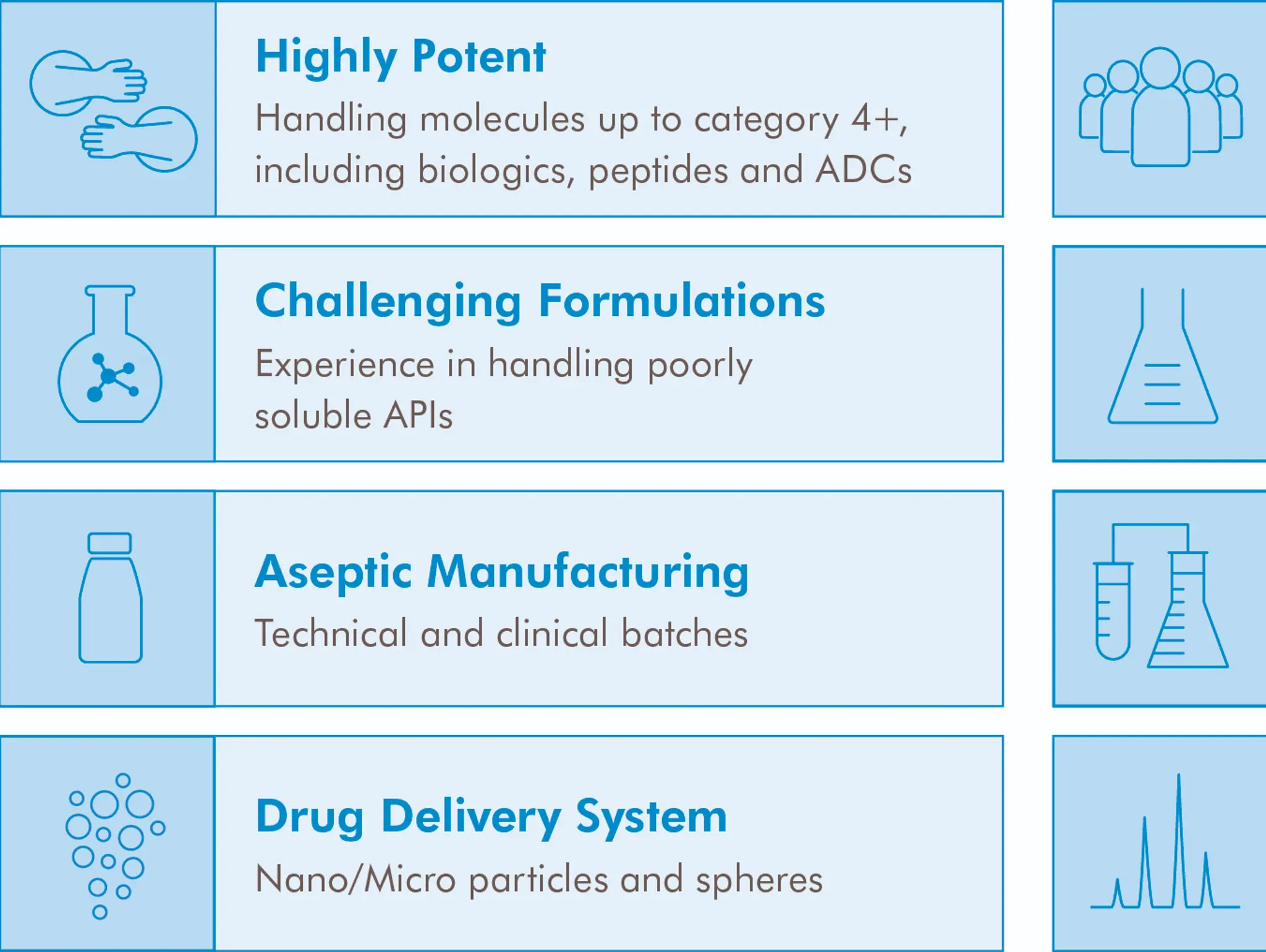
SPOTLIGHT: How CARBOGEN AMCIS supports complex molecules and challenging formulations from early development to commercialisation
30 September 2025
IPT highlights some of the most recent exciting advancements in pharmaceutical manufacturing
Product Profile, 30 September 2025
9 May 2025 -- Missouri, US -- Natoli Engineering, the global leader in tablet compression tooling, is unveiling the EZ Level Feeder Base Leveling System, Part Number SU 2970. This innovative design eliminates long setup times, enhances accuracy and minimizes formulation loss, and increases production time. You will no longer need to settle for “close enough” or “good enough” when using this solution that is built with precision in mind.
Product Profile, 8 May 2025
22 April 2025 -- Bubendorf, Switzerland -- CARBOGEN AMCIS, a Switzerland-based pharmaceutical process development and Active Pharmaceutical Ingredient (API) manufacturing company, is pleased to announce that its Shanghai facility has successfully obtained its first Drug Manufacturing License (DML) from China’s National Medical Products Administration (NMPA).
Product Profile, 21 April 2025
25 March 2025 -- Minnesota, US -- Central Research Laboratories (CRL), a global leader in the remote-handling industry, is launching Single-Use Gamma Bags that have been designed to undergo gamma-sterilization procedures. CRL Single-Use Gamma Bags are an addition to the company’s Single-Use Beta Bag product line, which also includes the recently launched Single-Use Double Bags.
Product Profile, 25 March 2025
A second edition of the LASER COMPONENTS Pyroelectric Receiver series is now available!
Product Profile, 11 March 2025
4 March 2026 -- Halle (Saale), Germany -- With the SONOFLOW CO.55 SD V3.0 flow meter, SONOTEC presents precision, process control, and uniqueness at the premiere of the Single-Use Event Basel on March 19, 2026, in Basel, Switzerland. Just one week later, bioprocess engineers will have the opportunity to experience the unique sensor family at GMP PharmaTechnica from March 24 to 25, 2026, in Wiesbaden, Germany.
Press Releases, 4 March 2026
24 February 2026 -- California, US -- Illumina today announced the launch of TruPath Genome, setting a new standard for high-quality, comprehensive whole genome insights for genetic disease. TruPath Genome has been found to deliver unparalleled accuracy and resolution—even across so-called “dark regions” of the genome—providing researchers with a more complete picture of genomic alterations implicated in genetic disease.
Press Releases, 24 February 2026
24 February 2026 -- London, UK -- PMGroup Worldwide Ltd are pleased to announce the acquisition of Laboratory News magazine and website, together with Laboratory Talk website, from Synthesis Media.
Press Releases, 24 February 2026
23 February 2026 -- Illinois, US -- AbbVie today announced a new $380 million investment to build two new active pharmaceutical ingredient (API) manufacturing facilities at its current North Chicago, Illinois, campus.
Press Releases, 23 February 2026
23 February 2026 -- Cambridge, UK -- Syndex Bio, a biotechnology company advancing next generation molecular diagnostics, today announced the introduction of its proprietary mcPCR (methyl copying PCR) platform at the Advances in Genome Biology and Technology (AGBT) conference in Orlando, Florida.
Press Releases, 23 February 2026
How is retrieval-augmented generation aiding smaller biotechs in collating and utilising disparate data systems?
Digital, 16 December 2025
What are co-folding models and how is their use transforming drug development?
Digital, 16 December 2025
How can pharma companies improve their digital systems while maintaining regulatory compliance, and ensure the two teams work together cohesively?
Digital, 16 December 2025
Artificial intelligence-powered de novo protein design is opening new pathways in biologics discovery, allowing researchers to create synthetic proteins beyond nature’s existing repertoire
Digital, 30 September 2025
How is artificial intelligence, when delivered through software as a service platforms, unlocking real business outcomes for pharmaceutical manufacturers, from faster batch release and smarter investigations to smoother tech transfers and enterprise-wide compliance?
Digital, 30 September 2025
How is semantic layering technology addressing significant efficiency gaps, creating the infrastructure for more efficient manufacturing operations?

Process innovations provide opportunities to reduce environmental impact while improving efficiency and product quality

How is X-ray CT being used to ensure quality in tabletting?

In biologics development, clone selection is not just a technical milestone, it’s a strategic decision with far-reaching clinical and commercial implications. How can thoughtful clone selection – integrated with developability and manufacturability assessments – accelerate speed to clinic while safeguarding long-term success?

Biocatalysis is enhancing complex molecule process development. By merging the power of enzymes with chemical synthesis, scientists and manufacturers are addressing long-standing challenges in stereoselectivity, route complexity and sustainability

SPOTLIGHT: How CARBOGEN AMCIS supports complex molecules and challenging formulations from early development to commercialisation
What are eight compelling reasons why manufacturers should prioritise the use of advanced contamination detection systems in their operations?

SPOTLIGHT: Bill Supplee at Natoli Engineering discusses the best ways to ensure tablet density uniformity in pharma products

Sustainability is top of the agenda for many pharmaceutical manufacturing companies. Which role do technology providers and their equipment play in reducing CO2 emissions

Why is the use of synthetic DNA a better choice for emerging genetic medicines?
What are some innovative techniques used for manufacturing gene therapies at a commercial scale?

Tablet formulation development often uses small R&D tablet presses to optimise both the composition of the formulation and manufacturing process parameters. These types of machines have a wide variety of capabilities and may be either single station or small rotaries.
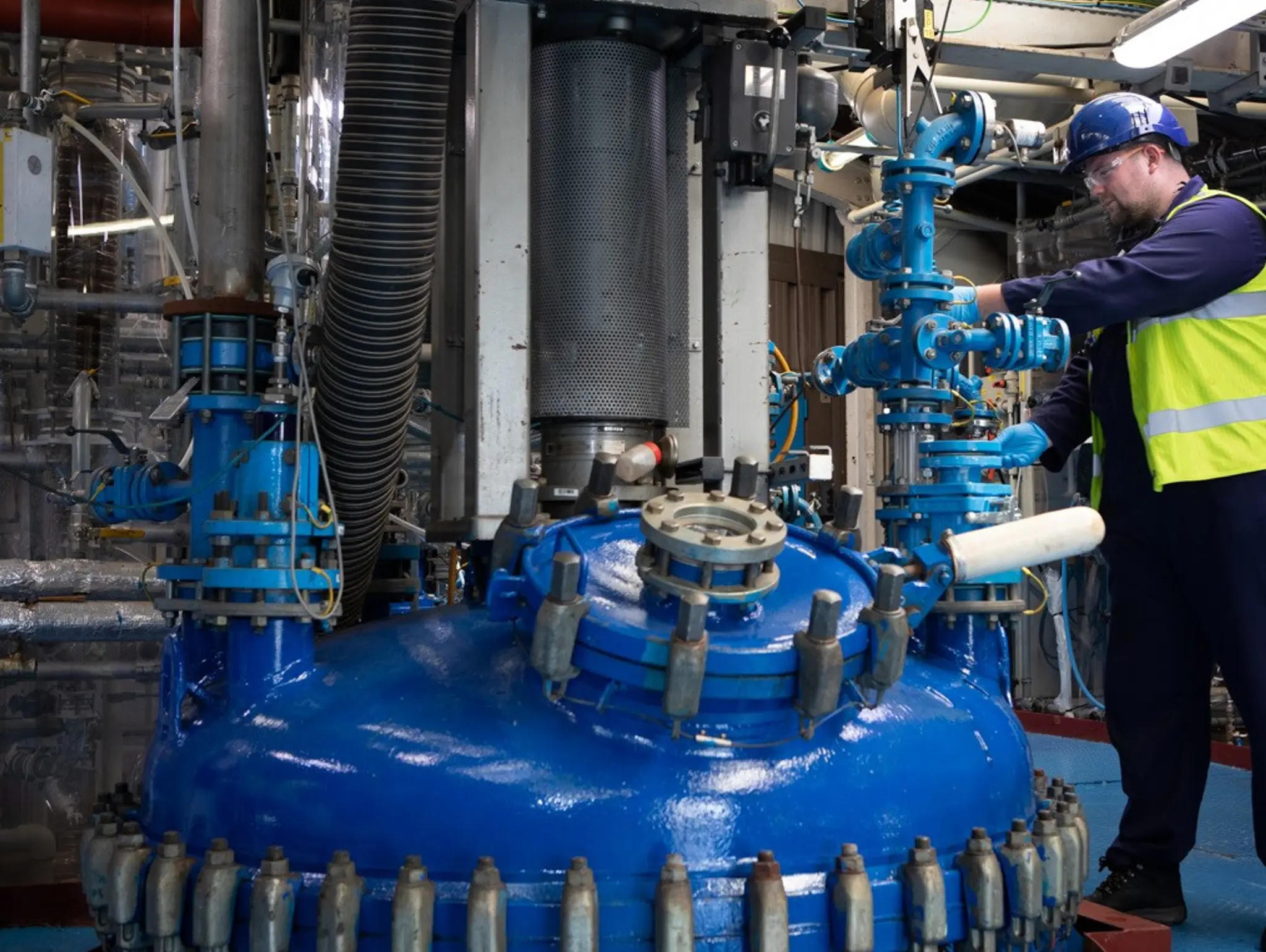
CARBOGEN AMCIS’ Manchester, UK, site plays a crucial role in early-phase drug development, providing high-quality starting materials and building blocks across industries, including pharmaceuticals, cosmetics, electronics and dental applications.
Our expertise in drug product development encompasses a wide range of pharmaceutical services. From formulation design to commercial-scale manufacturing, we specialise in efficiently bringing your drug product to market while ensuring full compliance with regulatory standards. Our highly skilled team at our state-of-the-art facility in Saint-Beauzire, France, ensures that your drug product is manufactured with precision and meets the highest quality standards.

With a strong track record in complex chemistry, CARBOGEN AMCIS offers end-to-end drug substance solutions, from preclinical development to commercial supply.
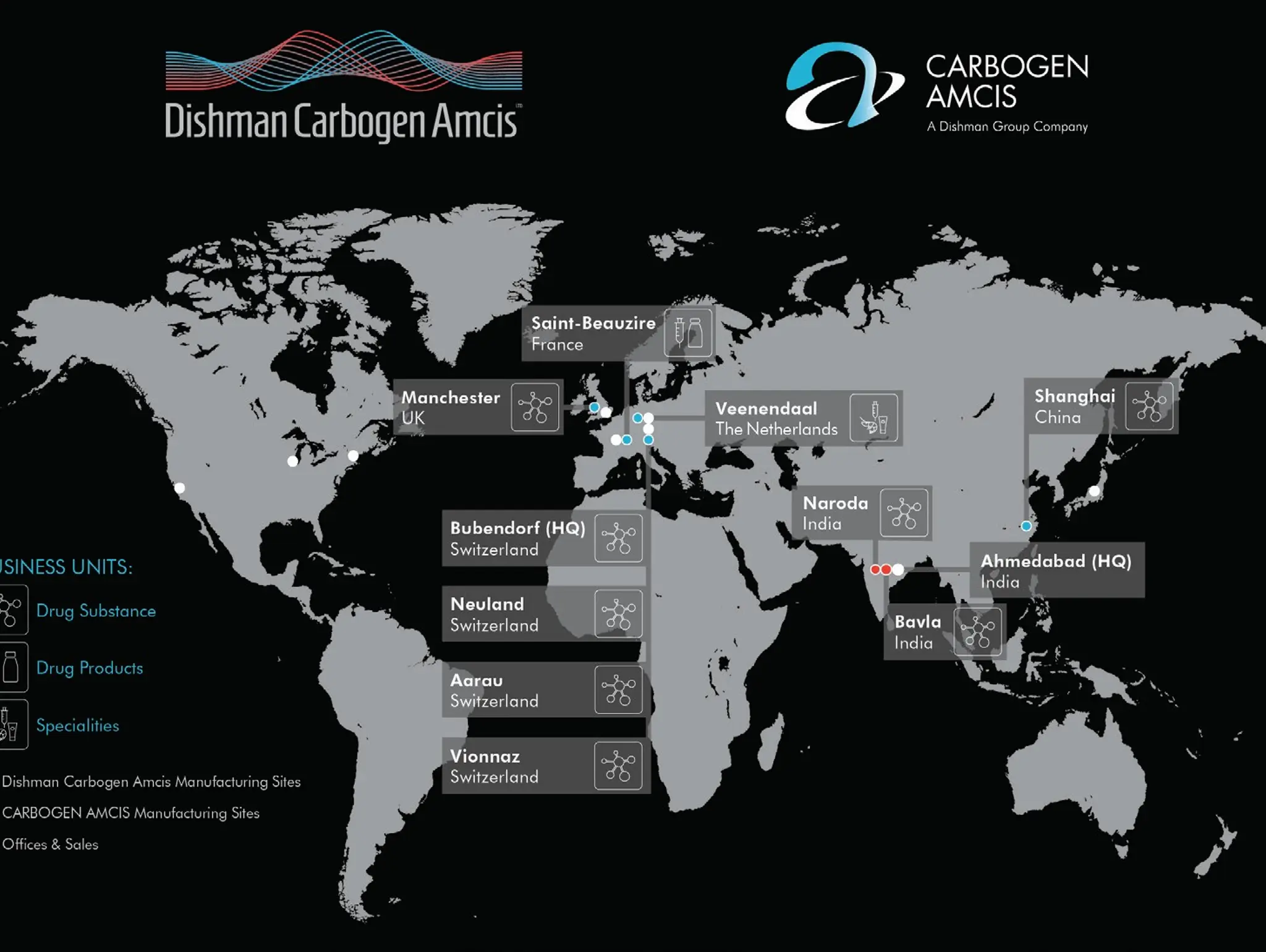
CARBOGEN AMCIS is a leading global contract development and manufacturing organisation (CDMO), trusted by pharmaceutical and biopharmaceutical companies worldwide. With over 40 years of expertise, the company offers an integrated suite of services across drug substance and drug product development, helping clients to bring life-changing therapies to market with precision and efficiency.

How is artificial intelligence being utilised in labs to synthesise design and workloads?

How are emerging design principles like operational insight, molecular infrastructure and human-centred design reshaping safety, agility and performance across the life sciences landscape?

How can the introduction of automation in fluid management systems improve biopharma manufacturing?

How are modern pharma labs being designed with practicality and connectivity to ensure streamlined processes and happy staff?

How is the industry translating the potential of automated technologies into practical, flexible and scalable workflows that address the real-world challenges of modern laboratories, ensuring that innovation delivers measurable scientific and operational gains?

As the need for ultra-low temperature sample storage rises, many labs are stuck expanding outdated ‘freezer farms’ to meet demand. High-density automated cold storage offers a smarter, more efficient solution, but reaping the benefits depends on more than the technology itself – it requires thoughtful change management. How can companies approach change management to drive lasting adoption and maximise the value of automation?

In an era of cost-conscious innovation, including tighter budgets, talent shortages and escalating regulatory demands, life sciences labs are under pressure to improve performance while managing budgets more wisely. By focusing on equipment utilisation and asset intelligence, labs can reduce downtime, avoid unnecessary purchases, optimise maintenance intervals and scale smarter – all without compromising quality or compliance

IPT talks to Roya Amini-Naieni at Trilobio about robotic automation in pharma research, the different applications of soft-and hardware, and how the field is likely to develop over the next five years

IPT talks to David Fuller at Artificial Inc about how automated labs are revolutionising the pharma R&D space by connecting disparate systems, easing the burden of manual tasks on workers, and speeding up time-to-market with tools such as artificial intelligence

Ensuring a manufacturing environment stays clear of bacteria and fungi is of utmost importance, especially when it comes to pharmaceuticals. How can this highly specialised monitoring be achieved in a fast, cost-effective manner?

A connected laboratory, where systems and devices communicate seamlessly, is the foundation to unlock AI’s full capabilities

With the lab of the future becoming increasingly automated, how will this change workflows and how can the industry prepare for this?

The pharmaceutical sector is characterised by intricate processes, stringent regulations and a constant demand for innovation. However, the industry's digital transformation is hindered by the prevalence of legacy machinery, which often poses compatibility challenges when deploying new technology. COPA-DATA explains the method of standardisation to remove the barriers of integration of legacy equipment in labs and production lines

'Increasing economic challenges like market vitality, product diversification and cost pressure lead to the need for faster process design' − and that is not to mention the shorter innovation cycles and increasing demand for small batch production. The module type package suite from COPA-DATA meets these needs

Lab layouts, until the introduction of automation, had seen little change in decades. However, the increased usage of hardware and software lab automation has reduced repetitive manual tasks and drastically increased the walkaway time involved in experiments
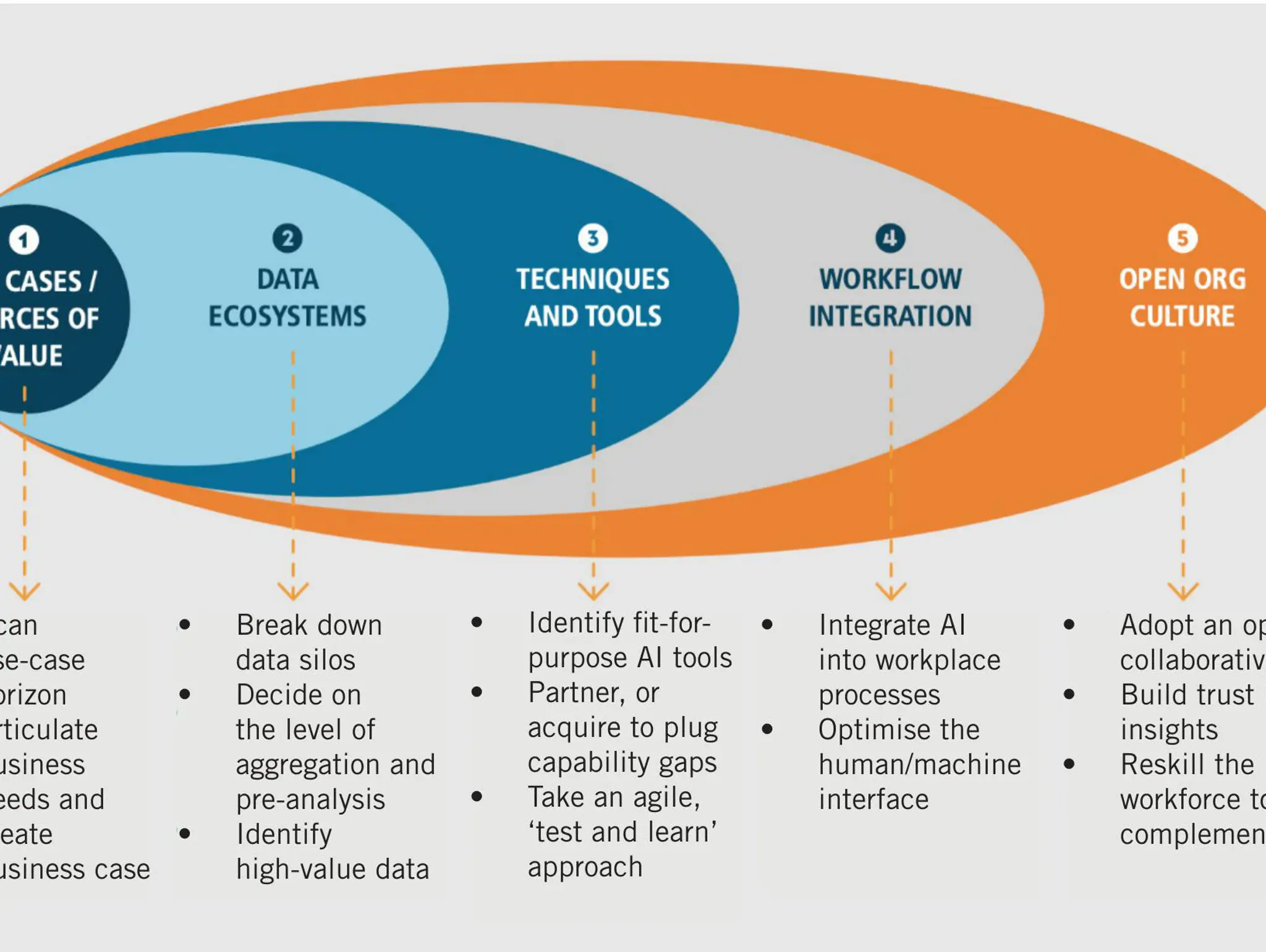
Whether lab managers are enthusiastic or apprehensive, AI is coming to the lab. The key to building this advantage is not in the technology, but the approach. Successful adoption of AI requires a genuine, long-term commitment. IPT spoke to Bob Voelkner at LabVantage Solutions about the implications of AI for pharma, what it takes to succeed with this new technology and his five recommended steps for getting started

Mapping disease cells in tissue is essential, but extracting and analysing them is what advances drug discovery. Spatial cell sorting enables physical isolation of individual cells for comprehensive multi-omics profiling – bridging the gap between tissue visualisation and functional validation
Discovery and Development, 16 December 2025
Nanotechnology has unlocked a new generation of hydrogels that can be tuned for specific clinical demands. From degradation kinetics to drug release and mechanical resilience, nanoscale control is transforming hydrogels from passive wound dressings into active, minimally invasive platforms for joint restoration, tissue regeneration and targeted therapeutics
Discovery and Development, 16 December 2025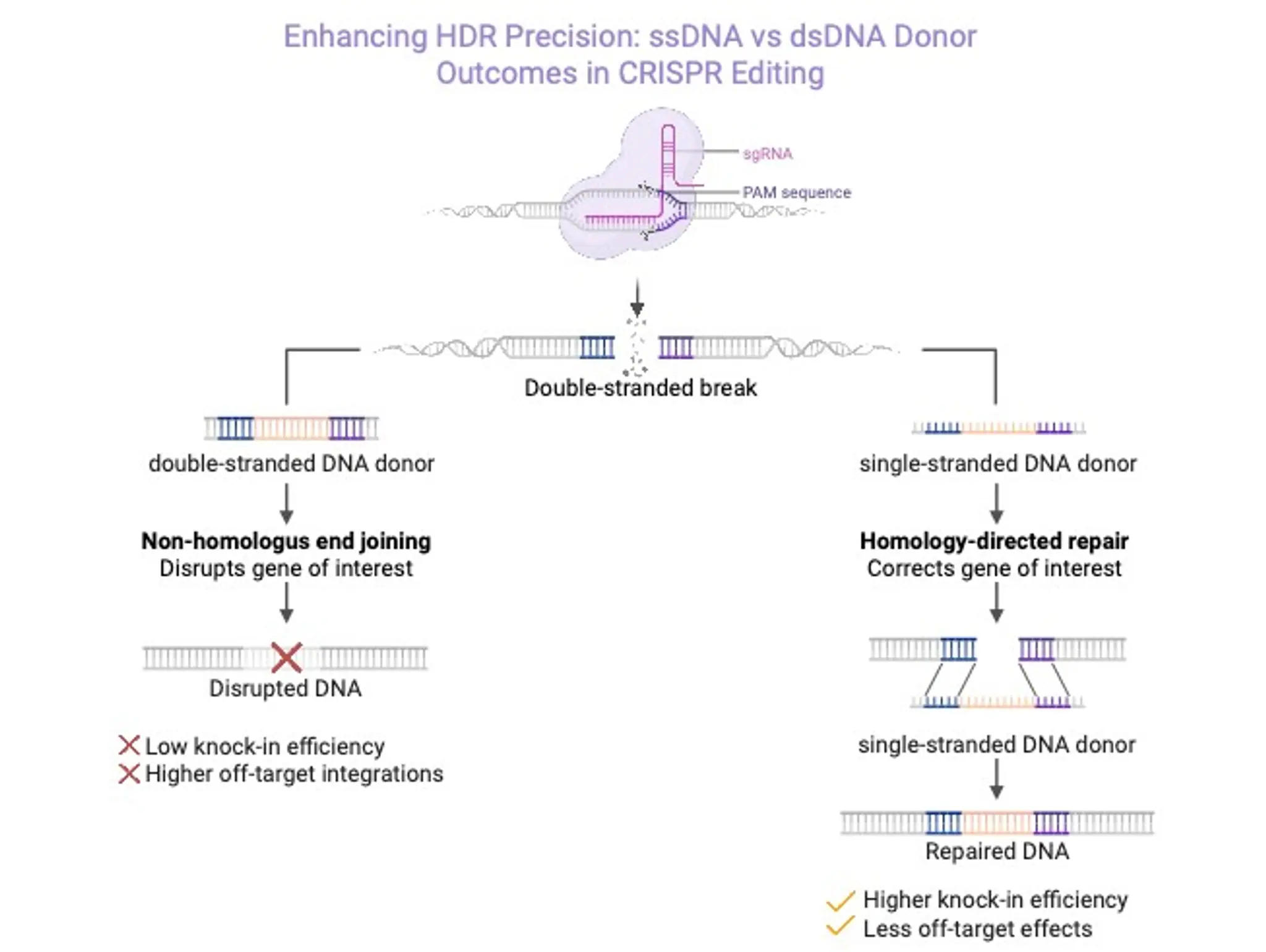
What is single-stranded DNA and why is it a viable viral vector alternative?
Discovery and Development, 30 September 2025
How will innovative human-centred in vitro testing strategies contribute to accelerating the delivery of safer and more effective therapies to patients without, or with minimal, use of animal models?
Discovery and Development, 30 September 2025
Should viral clearance studies be required for genetically engineered viral vectors and viral vector-derived products?
Discovery and Development, 30 September 2025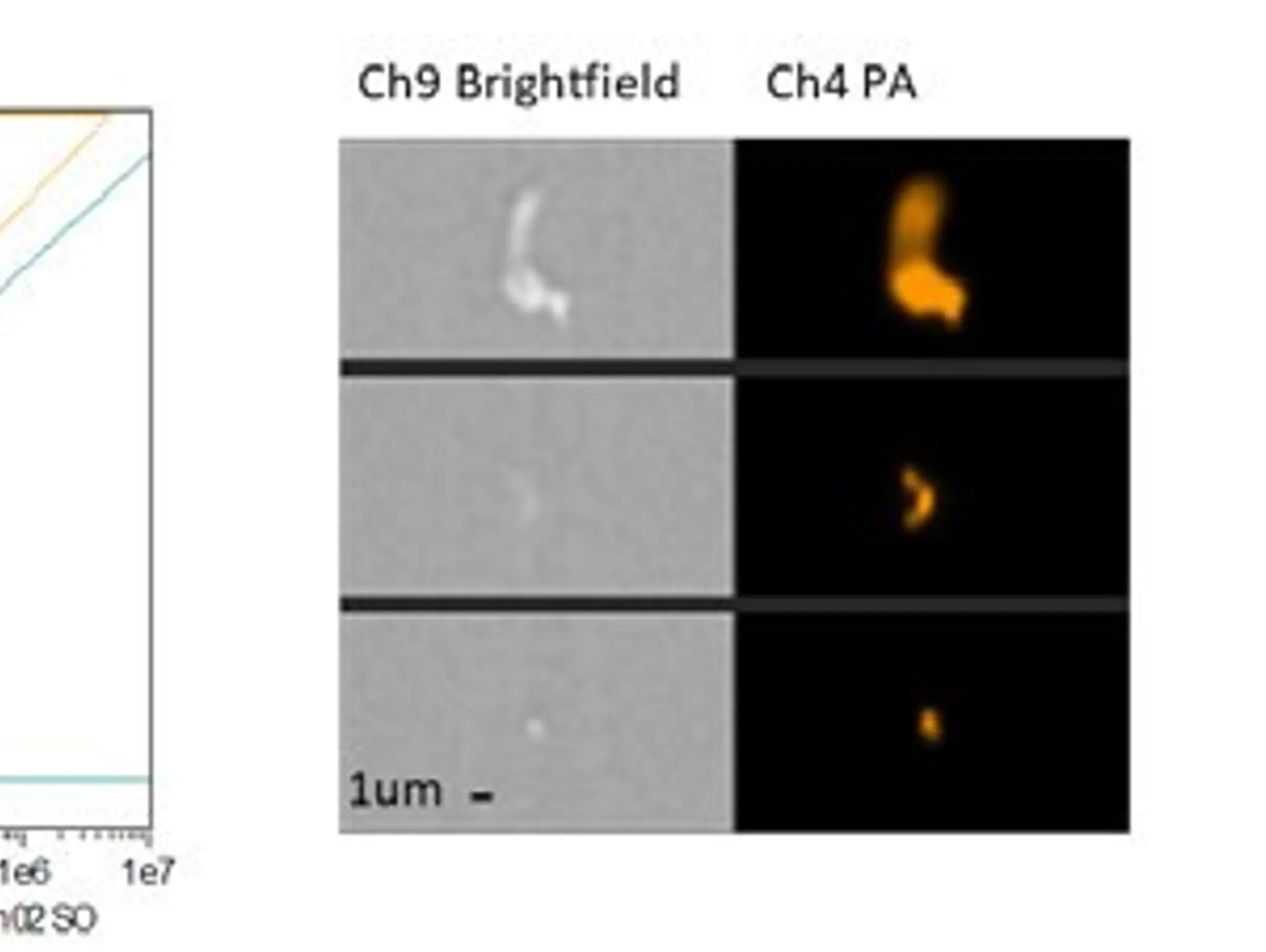
Advancements in imaging flow cytometry have improved the detection of particles in pharmaceutical formulations and helped provide insights into how these protein-based therapeutics work
Imaging & Sensing, 16 December 2025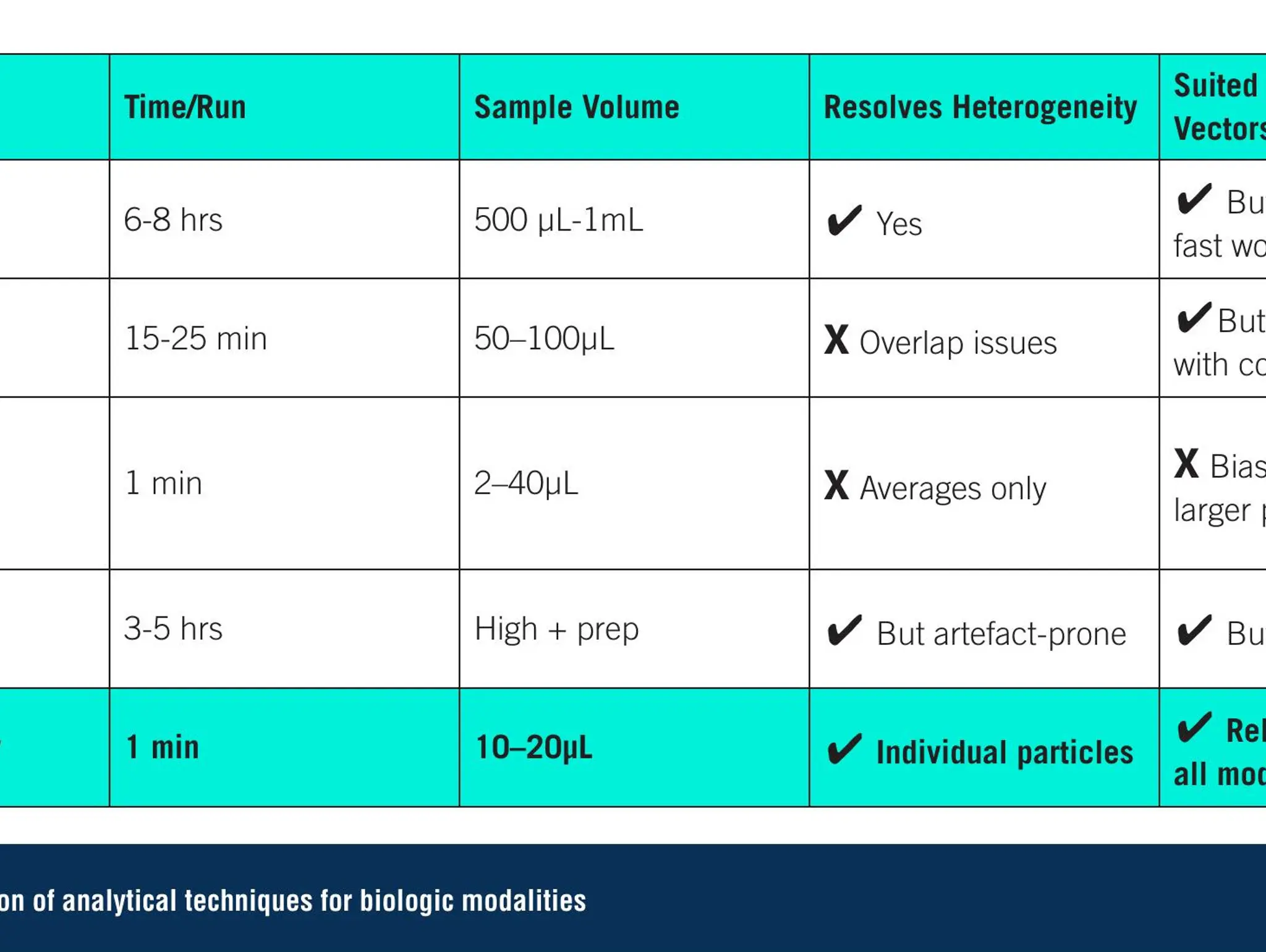
As novel biologics reshape the therapeutic landscape, more advanced analytics are essential. Single-particle technologies can help fill this gap, delivering faster, clearer insights and supporting the next generation of advanced therapies
Imaging & Sensing, 30 September 2025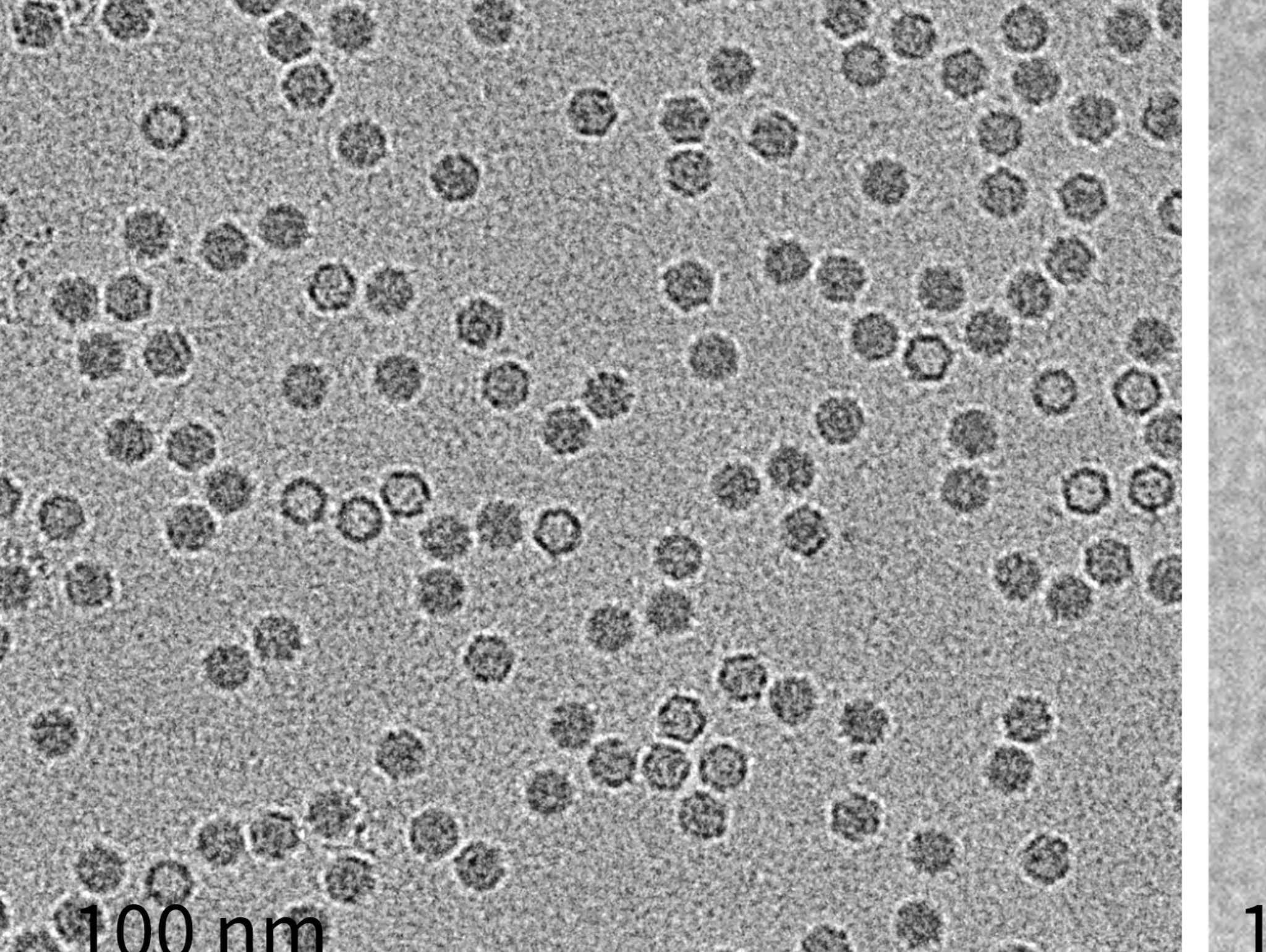
How is low voltage electron microscopy revolutionising the electron microscopy landscape – making the practice more accessible in pharma labs?
Imaging & Sensing, 30 September 2025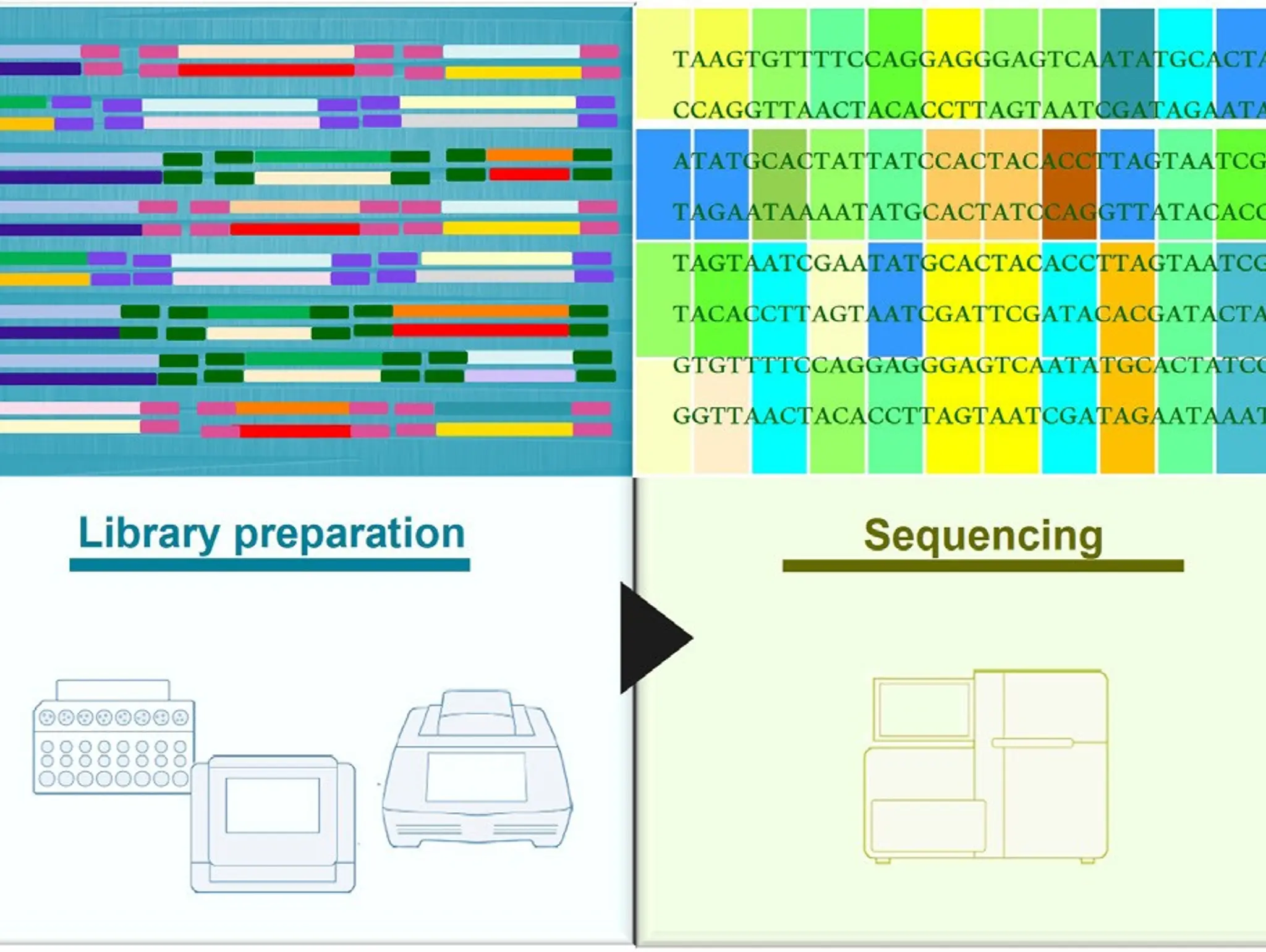
Massive sequencing, or next-generation sequencing, has opened the door to the fascinating world of genetics, and the secret on how to use this powerful tool most effectively hinges on the starting point: the sample
Imaging & Sensing, 30 September 2025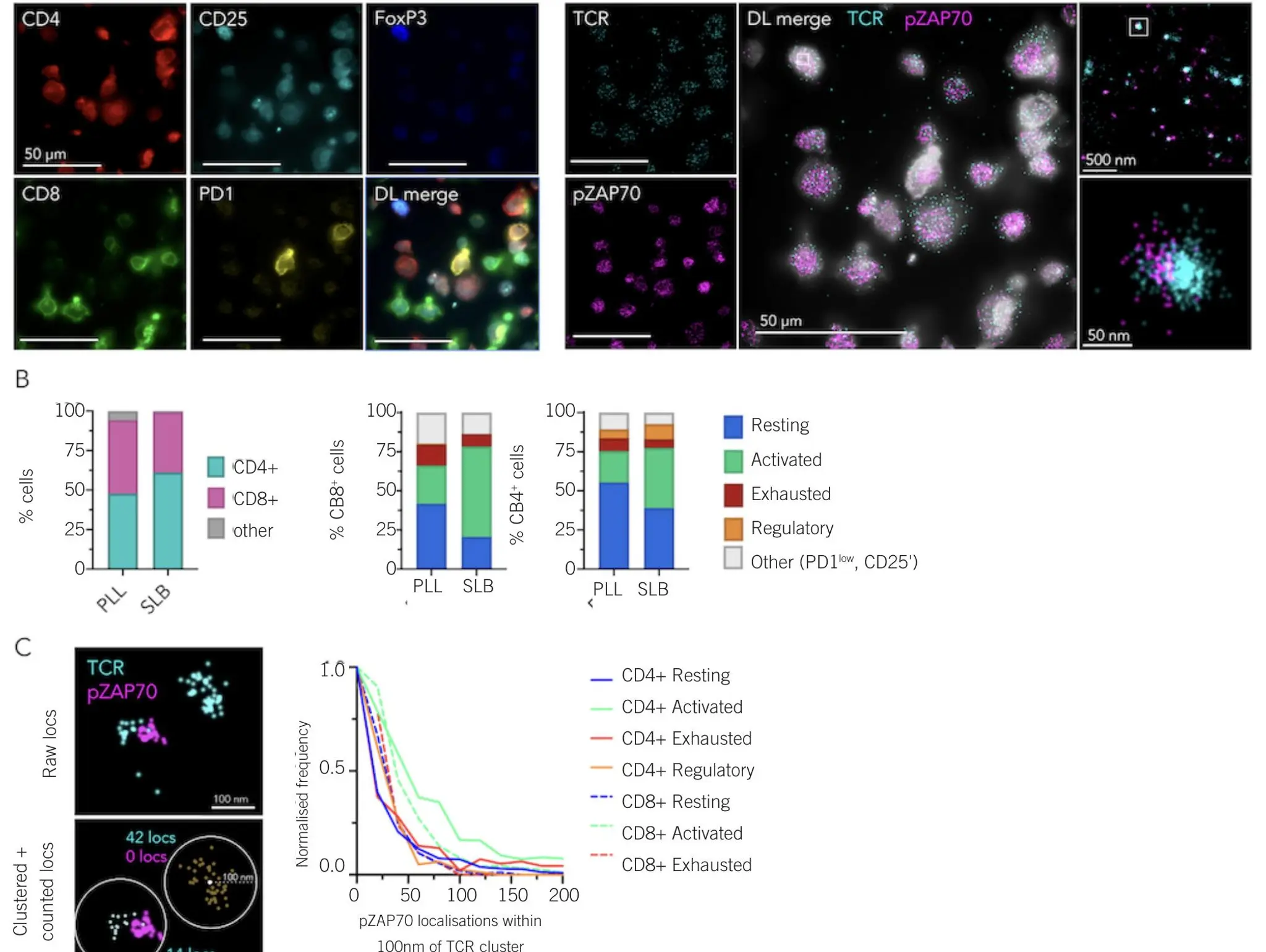
What is super-resolution microscopy and how is it accelerating progress across the imaging sector?
Imaging & Sensing, 30 September 2025
1-3 July 2025, Manchester, UK
Imaging & Sensing, 17 June 2025
11 February 2026 -- London, UK -- Over 350 delegates from leading and upcoming drug discovery and technology companies from the UK, Nordic and many other European regions, plus investment firms throughout Europe, will get together in London on 23rd April, 2026 for the 22nd Annual Anglonordic Life Science Conference
Conferences and Events , 11 February 2026
10 July 2025 -- Vienna, Austria -- BIO-Europe, Europe's premier biopharma partnering event, is heading to Vienna, Austria on November 3-5, 2025, followed by Digital Partnering on November 11-12.
Conferences and Events , 9 July 2025
2 June 2025 — London, UK — In just two weeks, the second edition of the London Biotechnology Show (LBS) returns to ExCeL London on 18–19 June, bringing together one of the most influential gatherings in the global biotechnology calendar. The event promises cutting-edge science, transformative innovation, and forward-thinking leadership.
Conferences and Events , 1 June 2025
World Vaccine Congress Europe will host 2500+ attendees, 280 speakers, 150 exhibitors and start-ups over the course of 4 days. With a multitude of tracks, the congress will cover everything vaccine related from start-to-finish from 13-16 October 2025.
Conferences and Events , 26 May 2025
Join global biotech and pharma leaders in Singapore, September 9–10, 2025, for the third edition of Asia Bio Partnering Forum.
Conferences and Events , 22 May 2025
Inside the 2024 American Biomanufacturing Summit
IPT TV, 3 June 2025
Join the UK’s leading biotechnology event to explore groundbreaking biotech innovations, connect with global healthcare leaders, and discover the future of life sciences for 𝐅𝐑𝐄𝐄.
IPT TV, 21 April 2025
Come and join us for the 13th edition of BioFIT, on December 3rd & 4th, 2024 in Lille and digital meetings days on December 11th & 12th, 2024!
IPT TV, 9 October 2024
Showcasing innovation, accelerating investment and facilitating partnering for life science leaders in Northern Europe.
IPT TV, 8 September 2024
Accelerate Your Therapeutics to Market: Expedite R&D, Improve CMC Efficiency & Build New Partnerships Join the industry’s leading event in Europe and access new data & case studies from world-renowned speakers and companies across the entire landscape of oligonucleotides, peptides, mRNA, drug delivery and partnering strategies -- from discovery to market.
IPT TV, 4 September 2024